Research carried out at the São Carlos Institute of Chemistry at the University of São Paulo (IQSC-USP) resulted in a nanostructured material that works as a catalyst for electrochemical reactions (electrocatalyst) that are fundamental in some renewable energy generation systems. As it combines efficiency and low cost, the new material would be an alternative to the catalysts traditionally used in these reactions, which are based on elements of the group of precious metals, such as platinum, which are scarce and expensive.
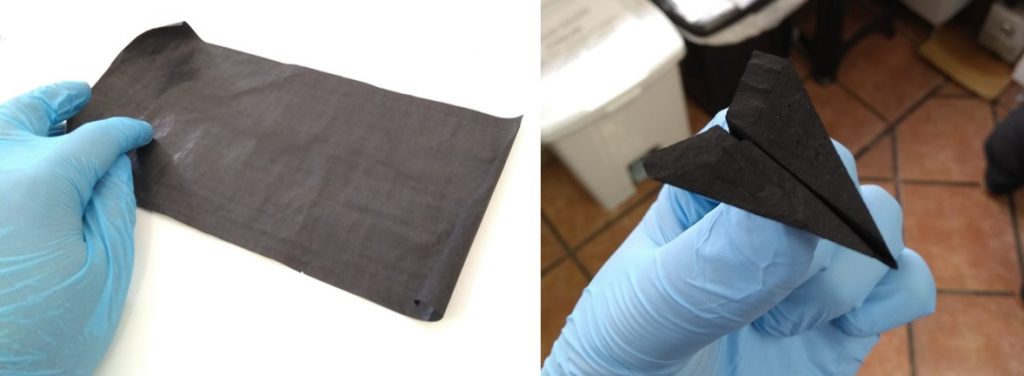
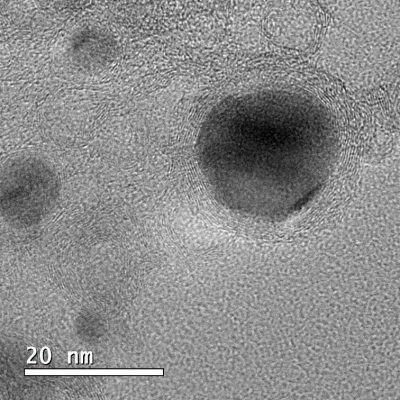
The developed material, which, with the naked eye, has the appearance of a black powder, is hybrid and nanostructured. It consists of nanoparticles from 10 to 50 nm, composed of an iron, cobalt and nickel alloy (three relatively abundant and cheap elements), inserted in layers of carbon doped with nitrogen.
Recently reported in the Journal of Materials Chemistry A, the study presents a very simple process to obtain this material with the necessary stability for electrocatalysis applications. The method consists of preparing a water solution with iron, cobalt and nickel salts and adding organic compounds capable of binding metal ions (so-called ligands). The reaction between metals and ligands generates structures known as MOFs (metal-organic frameworks). Eventually, the obtained MOFs are submitted to high temperature (900 ° C) to obtain the final material.
“We have come up with a unique straightforward yet effective strategy for synthesis of an efficient electrocatalyst that is cheap and quite active in diver’s energy conversion reactions and could have impact in new generation energy related technologies,” says Mohmmad Khalid, a postdoctoral fellow at the Electrochemistry Group at IQSC-USP and corresponding author of the article with Professor Hamilton Varela (IQSC-USP).
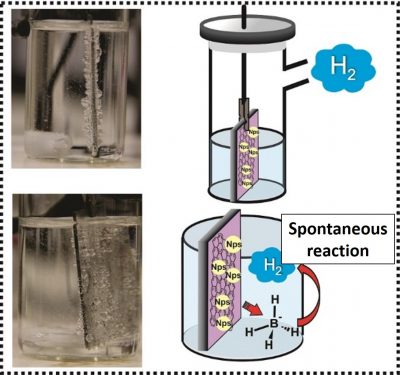
The article also reports the tests carried out at the laboratories of the Electrochemistry Group at IQSC-USP to assess the performance of the nanostructured material in some applications related to sustainable energy generation, such as the division of the water molecule (hydrolysis). This process is the cleanest way to obtain hydrogen, currently considered the most promising non-fossil fuel. However, without the participation of good electrocatalysts, hydrolysis is very slow and consumes a lot of electricity. “Our nanostructured catalyst in overall water splitting impeccably works for decomposing apart the water molecules for the generation of hydrogen at applying very low potential compare to several previously reported nonprecious electrocatalysts,” says Khalid.
The nanostructured material also showed very good results as a catalyst for ethanol oxidation. This reaction is carried out on direct ethanol fuel cells to obtain electrical energy from the chemical energy of ethanol (renewable fuel with Brazil as the second largest producer in the world). “Thus, the catalyst showed its potential not only to generate hydrogen, but also for fuel cell applications,” says Khalid.
Overcoming the challenges
The work began in 2017, with a research project coordinated by Professor Hamilton Brandão Varela de Albuquerque, with the participation of postdoctoral fellow Mohmmad Khalid. According to Khalid, the final objective of the study was to find a cheap and stable electrocatalyst for the process of dividing the water molecule.
The main problems the researchers faced were the aggregation of nanoparticles during the synthesis of the material and its dissolution in the electrolytes during the electrochemical tests. “The interesting idea came up with brain-storming discussion of Dr. Ana Maria Borges Honorato and after multiphases optimizing conditions of synthesis process,” says Khalid. In the material obtained, the carbon layers protect the catalyst nanoparticles and influence the material’s catalytic performance, which is affected by the thickness of these layers and by small variations in their composition. “This nanostructure allowed us to solve not only the problem of particle aggregation during synthesis and the problem of metal segregation/dissolution in electrolytes during the operation, but also to improve the catalytic performance in oxygen reduction, oxygen evolution, hydrogen evolution, ethanol oxidation reactions and general water division, with very competitive values in relation to reference catalysts,” summarizes the postdoctoral fellow.
The work received funding from Brazilian agencies CAPES, CNPq and FAPESP (São Paulo).

[Paper: Trifunctional catalytic activities of trimetallic FeCoNi alloy nanoparticles embedded in a carbon shell for efficient overall water splitting. Mohd. Khalid, Ana M. B. Honorato, Germano Tremiliosi Filho and Hamilton Varela. J. Mater. Chem. A, 2020,8, 9021-9031.]

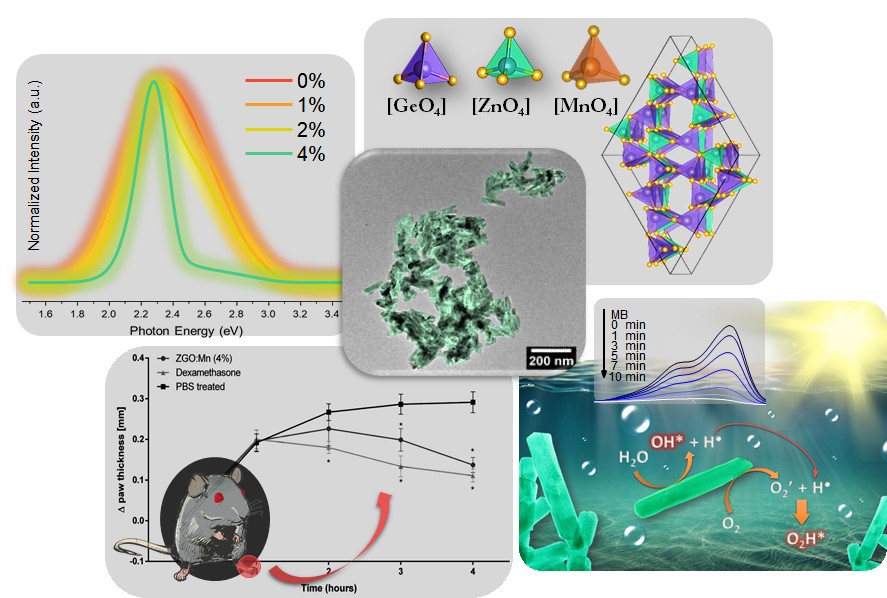
 A team of researchers from Brazilian universities found, in cylindrical nanostructures known as nanorods, an anti-inflammatory effect equivalent to that achieved by commercial drugs. Researchers have also demonstrated the effectiveness of these nanorods as catalysts (accelerators) in the degradation of a pollutant. These applications are even more relevant considering that the scientific team was able to produce large quantities of the material through a simple and fast process. The work carried out shows the potential of these nanorods for the development of new medicines and for the treatment of effluents.
A team of researchers from Brazilian universities found, in cylindrical nanostructures known as nanorods, an anti-inflammatory effect equivalent to that achieved by commercial drugs. Researchers have also demonstrated the effectiveness of these nanorods as catalysts (accelerators) in the degradation of a pollutant. These applications are even more relevant considering that the scientific team was able to produce large quantities of the material through a simple and fast process. The work carried out shows the potential of these nanorods for the development of new medicines and for the treatment of effluents.