[Paper: Characterization of the structural, optical, photocatalytic and in vitro and in vivo anti-inflammatory properties of Mn2+ doped Zn2GeO4 nanorods. Suzuki, V. Y.; Amorin, L. H. C; Lima, N. M; Machado, E. G; Carvalho, P. E.; Castro, S. B. R.; Souza Alves, C. C.; Carli, A. P.; Li, Maximo Siu; Longo, Elson; Felipe La Porta. J. Mater. Chem. C, 2019, 7, 8216. DOI: 10.1039/c9tc01189g]
 A team of researchers from Brazilian universities found, in cylindrical nanostructures known as nanorods, an anti-inflammatory effect equivalent to that achieved by commercial drugs. Researchers have also demonstrated the effectiveness of these nanorods as catalysts (accelerators) in the degradation of a pollutant. These applications are even more relevant considering that the scientific team was able to produce large quantities of the material through a simple and fast process. The work carried out shows the potential of these nanorods for the development of new medicines and for the treatment of effluents.
A team of researchers from Brazilian universities found, in cylindrical nanostructures known as nanorods, an anti-inflammatory effect equivalent to that achieved by commercial drugs. Researchers have also demonstrated the effectiveness of these nanorods as catalysts (accelerators) in the degradation of a pollutant. These applications are even more relevant considering that the scientific team was able to produce large quantities of the material through a simple and fast process. The work carried out shows the potential of these nanorods for the development of new medicines and for the treatment of effluents.
The work originated about three years ago when Professor Felipe de Almeida La Porta, who had recently joined the faculty of the Federal Technological University of Paraná (UTFPR), Londrina campus, was implementing a research group on nanotechnology and computational chemistry at this university. “Our laboratory was investigating some classes of emerging materials, with the perspective of aligning theory and practice, thus driving new discoveries and applications,” says La Porta. One of the materials studied by the group was zinc germanate (Zn2GeO4), a versatile semiconductor with well-known applications in sensors, catalysts, batteries and other devices.
Together with undergraduate researcher Victor Yuudi Suzuki, the professor started a project in which he synthesized pure Zn2GeO4 nanorods at the UTFPR laboratory with very small percentages of manganese ions. To produce this series of nanorods, they used “microwave assisted hydrothermal synthesis.” The method consists, in broad lines, of mixing aqueous solutions containing certain compounds, heating the final solution in a microwave oven and allowing the compounds to react for a certain period of time at controlled pressure and temperature. In this study, the manganese ion-doped Zn2GeO4 was prepared, and the reactions were performed at 140 °C for 10 minutes. The resulting material from these reactions was collected at room temperature, then washed and dried, which generate the nanorods.
Professor La Porta and his research group were able to optimize one of the process steps, the crystallization of materials, thus reducing the synthesis time from hours to a few minutes, but maintaining the quality of the material and the possibility to control its shape.
After preparing the samples, they traveled from Londrina (state of Paraná) to São Carlos (São Paulo state) to characterize the materials at the Center for Functional Materials Development (CDMF) at the Federal University of São Carlos (UFSCar) and at the Institute of Physics at the University of São Paulo (USP). Together with the local researchers, they were able to analyze the shape, structure and luminescence of the four types of nanorod compositions produced: manganese-free and with 1, 2 and 4% of this element incorporated into the structure of Zn2GeO4.
Finally, knowing that compounds containing zinc, germanium or manganese exhibit considerable effects on living things, the team contacted some collaborators to investigate these properties in the nanorods. Thus, several experiments were performed at the Departments of Chemistry and Pharmacy of the Federal University of Juiz de Fora and at the Federal University of Vales do Jequitinhonha and Mucuri, both in the state of Minas Gerais.

To study the anti-inflammatory action, the team performed in vitro tests (in contact with cells in laboratory containers) and also in vivo tests (using rats with paw edema, within the norms of the Brazilian code for laboratory animal use). Both types of experiments revealed that nanorods with about 4% manganese were the most effective in controlling inflammation. The in vitro tests showed these nanostructures were able to modulate molecules that regulate inflammation without causing cell death (without cytotoxicity). In the in vivo experiments, the nanorods reduced the induced rat paw edema with results similar to that of the application of dexamethasone, a well-known drug of the corticoid group.
“At first, we thought that combining these elements to form a ternary oxide could somehow potentiate these effects. But we had no idea the results would be so significant. Given that the drugs currently available in therapy are proving to be less effective every day, these results may encourage the use of these nanorods, for example in the production of a new pharmaceutical formulation, especially for cases of inflammation,” says Felipe La Porta, who is the corresponding author of the paper that was recently published by the research team in the Journal of Materials Chemistry C (impact factor 6,641).
In addition to proving the potential of the material for this application in the health area, the authors of the paper have experimentally verified the ability of nanorods to degrade a chemical dye widely found in industrial effluents, known as methylene blue. For this application, 2% manganese nanostructures were the most efficient, completely decomposing the dye in 10 minutes. “Due to the manufacture simplicity of this system, coupled with its excellent properties, this material is also promising for cleaning various environmental pollutants, and can be easily recovered at the end of this process,” adds Prof La Porta.
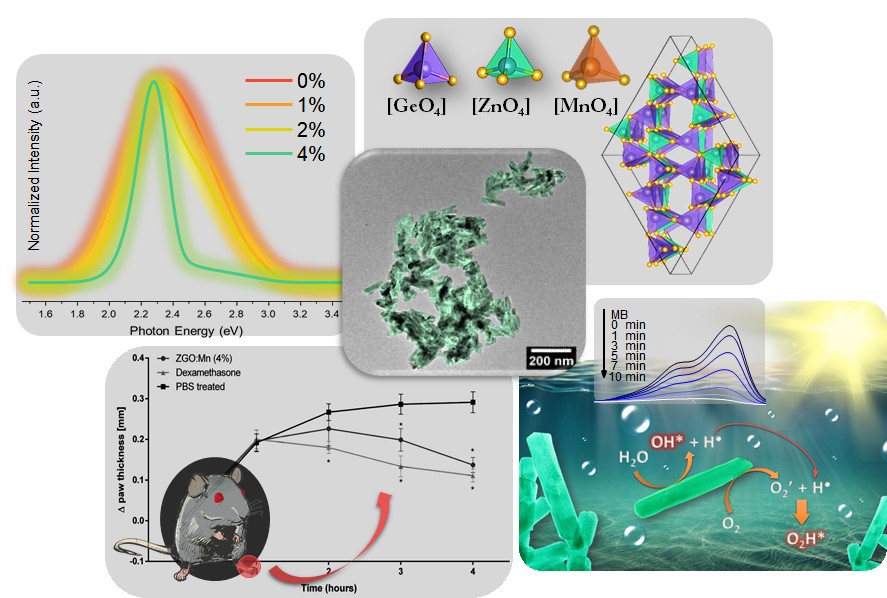
The superior properties that the Brazilian scientific team found in the nanorods with manganese can be related to the structural defects observed in these samples. In fact, the three-dimensional network of atoms that forms zinc germanate is crystalline, that is, organized in regular patterns. The introduction of manganese generates irregularities, and new properties emerge.
The scientific paper that reports this work was selected to be part of the Materials and Nano Research in Brazil collection, prepared by the Royal Society of Chemistry in celebration of the 18th B-MRS Meeting, and can therefore be accessed free of charge until October 15 of this year, here.
The work was carried out with funding from Brazilian research support agencies: the federal CNPq and Capes, and the state Araucaria Foundation, Fapesp and Fapemig.


