Unlike other solar cells that have dominated the market for a long time, such as silicon cells, the organic ones are thin, light, flexible and semi-transparent. With these characteristics, they become very attractive for specific segments. In Brazil, for example, which has national production, some of the largest installed surfaces in the world can be seen in business buildings, as well as some installations in shopping centers, trucks and bus stops.
Although the organic version of solar cells also offer advantages in large-scale production (simpler industrial processes with lower carbon footprint, such as the roll-to-roll), conquering big markets largely depends on an ongoing efficiency improvement to convert sunlight into electricity. To overcome this challenge, it is essential to develop materials with suitable properties and to combine different materials within the device.
A scientific team from the Brazilian Federal University of Paraná (UFPR) studied in detail, using experimental and theoretical tools, the charge generation mechanism in organic solar cells – a complex process that is not yet fully understood. In practice, the results of this work help choosing which materials should be used and how they should be synthesized, so that their properties enhance the efficiency in converting light into electricity. The research paper was reported in the Journal of Materials Chemistry C (impact factor 7.059), where it was highlighted on the back cover.
Unraveling the exciton dissociation
In the sandwich of layers that forms solar cells, the active layer (responsible for absorbing light and generating electric charges) is composed of semiconductor materials that, for organic devices, are polymers or other carbon-based molecules. When excited by light, these materials do not generate free electric charges, as is the case with inorganic semiconductors. They generate excitons, which are electron–hole pairs connected by forces of attraction between the negative charge of the first and the positive charge of the second.
In order to generate free charges, which form the electric current, it is necessary to break this connection, in a phenomenon called exciton dissociation. One way to achieve this is to create, in the active layer, an interface between an electron donating material and an electron acceptor. “Depending on the combination of these two materials, exciton dissociation processes can occur at a very low time scale, resulting in a more efficient charge generation,” explains Leandro Benatto, corresponding author of the paper. “However, this process is still not well understood,” he adds.
In their work, Leandro and the other authors focused specifically on trying to understand the exciton dissociation and the generation of free charges at the interface between the donor and acceptor material. The team carried out photoluminescence experiments, which are generally used to measure the efficiency in generating free charges in systems of this type, and developed a mathematical model that simulates the process. The experimental and theoretical results were very similar, proving the model’s accuracy. “We developed a model that simulates the kinetics of the process, including the several stages of exciton dissociation and considering the main characteristics of the interface,” he says. “Based on the kinetic model, it was possible to reproduce the experimental results in a comprehensible manner and more clearly observe the main factors that influence the efficiency of the free charge generation process in donor/acceptor interfaces,” he adds.
Fullerenes vs. Non Fullerenes
The study that produced the article was coordinated by two professors from the Physics Department of UFPR, Marlus Koehler and Lucimara Stolz Roman, who have a longstanding partnership in the theoretical and experimental study of organic solar cells. “The theoretical part began to be developed in 2019, at the end of my PhD in Physics at UFPR under the guidance of Professor Marlus, and continued in my postdoctoral work at the Nanostructured Devices Laboratory (DINE) under the coordination of Professor Lucimara,” says Leandro. Also participating in the research were Maiara de Jesus Bassi, PhD student in Physics in the group of Professor Lucimara, and Luana Cristina Wouk, PhD in Physics who was also under the supervision of Professor Lucimara Roman, and currently working at CSEM Brazil, a private applied research center, which helped contextualize the problem in the large-scale development scenario.
The initial idea of the work was to understand the difference between two types of electron acceptor molecules: those derived from fullerene (a carbon allotrope), which have excellent performance in the collection and transport of electrons but have a limited spectrum of light absorption, and compounds not derived from fullerenes, which in recent years have optimized the collection and transport properties. “This is a very interesting topic since, recently, the efficiency of organic solar cells based on non-fullerenes surpassed the efficiency of those based on fullerenes, although, a few years ago, it could not be imagined that fullerenes would be surpassed,” reports Leandro. “Currently, laboratory produced organic solar cells based on non-fullerenes have reached 18% efficiency,” he adds.
This research received funding from Brazilian agencies Capes, CNPq and FAPEMIG, INCT–Nanocarbono and COPEL (Companhia Paranaense de Energia).

[Paper: Kinetic model for photoluminescence quenching by selective excitation of D/A blends: implications for charge separation in fullerene and non-fullerene organic solar cells. L. Benatto, M. de Jesus Bassi, L. C. Wouk de Menezes, L. S. Roman and M. Koehler. J. Mater. Chem. C, 2020,8, 8755-8769].

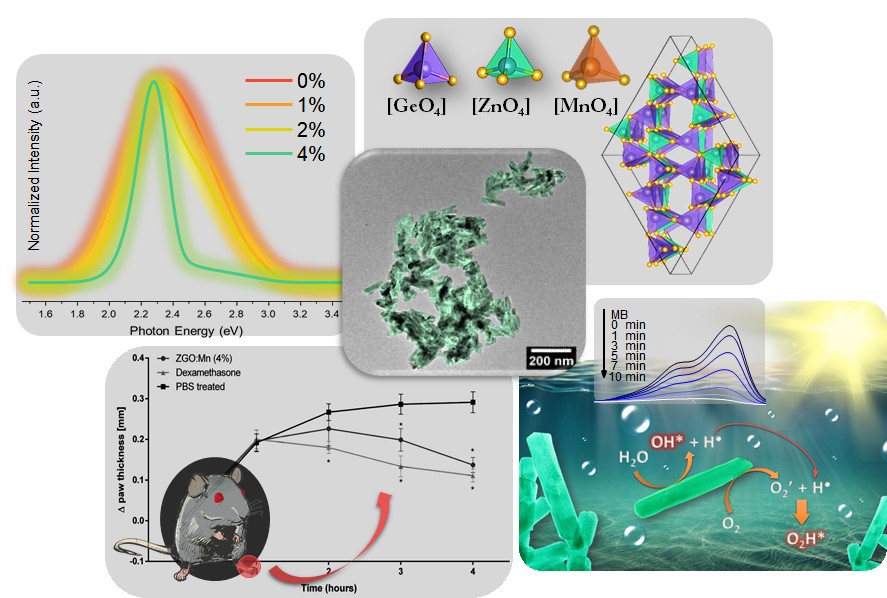
 A team of researchers from Brazilian universities found, in cylindrical nanostructures known as nanorods, an anti-inflammatory effect equivalent to that achieved by commercial drugs. Researchers have also demonstrated the effectiveness of these nanorods as catalysts (accelerators) in the degradation of a pollutant. These applications are even more relevant considering that the scientific team was able to produce large quantities of the material through a simple and fast process. The work carried out shows the potential of these nanorods for the development of new medicines and for the treatment of effluents.
A team of researchers from Brazilian universities found, in cylindrical nanostructures known as nanorods, an anti-inflammatory effect equivalent to that achieved by commercial drugs. Researchers have also demonstrated the effectiveness of these nanorods as catalysts (accelerators) in the degradation of a pollutant. These applications are even more relevant considering that the scientific team was able to produce large quantities of the material through a simple and fast process. The work carried out shows the potential of these nanorods for the development of new medicines and for the treatment of effluents.