[Paper: High-yield synthesis of bundles of double- and triple-walled carbono nanotubes on aluminum flakes. Thiago H.R. da Cunha, Sergio de Oliveira, Icaro L. Martins, Viviany Geraldo, Douglas Miquita, Sergio L.M. Ramos, Rodrigo G. Lacerda, Luiz O. Ladeira, Andre S. Ferlauto. Carbon 133(2018) 53-61.]
Aluminum flakes to produce carbon nanotubes

A team of scientists from institutions in Minas Gerais made a promising contribution to the production of carbon nanotubes. These hollow cylinders, whose carbon walls are only 1 atom thick, are already part of some products (batteries, automotive materials, water filters), but their industrial production is still incipient and needs solutions to lower costs and to increase efficiency, among other challenges.
The Brazilian researchers introduced a novelty in a stage of one of the most consolidated techniques for the mass production of nanotubes, chemical vapor deposition (CVD). As a result, the team was able to produce double- and triple-walled nanotube bundles (somewhat similar to two or three hollow cylinders, one inside the other). Thin, long and of high purity, the nanotubes had diameters of 3 to 8 nanometers, lengths up to 50 thousand times the diameter (from 150 to 300 micrometers) and 90% of carbon in their composition.
“The main contribution of this work is the presentation of a scalable and cost effective process for the synthesis of carbon nanotube bundles with large surface area (625 m2/g) and aspect ratio (50000:1),” says Thiago Henrique Rodrigues da Cunha, researcher of the Nanomaterials Technology Center (CTNano) of the Brazilian Federal University of Minas Gerais (UFGM) and corresponding author of this paper, which was recently published in the journal Carbon (impact factor 2017 = 7,082).
The method, in addition to generating good quality nanotubes, allows producing relatively large quantities of this material using relatively low amounts of raw materials. “Even using small systems, it is possible to obtain carbon nanotubes at a kilogram/day scale,” says the researcher. As the nanotubes obtained showed a very large ratio between surface area and mass (more than 625 square meters weighing only one gram), the production of nanotubes by this method could reach a few million square meters per day.
With the nanotubes obtained and a type of alcohol, the scientific team prepared a paste which was distributed over filter paper, forming a film that was separated from the paper when the paste dried. The black film was 40 micrometers thick and was flexible and foldable. Macroscopic aggregates of carbon nanotubes like this are commonly called buckypapers.
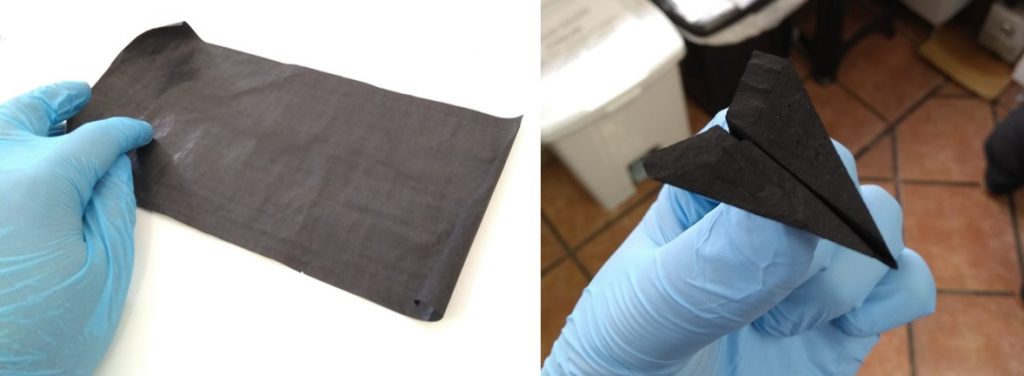
“The buckypaper produced from these nanotubes exhibited great surface area and good electrical conductivity, which makes them particularly interesting in the manufacture of electrodes for batteries and supercapacitors,” says Thiago da Cunha, who adds that the CTNano team is already working to use the buckypapers in these energy storage devices. A patent on the process was deposited at the end of 2017. “Our intention is to introduce this technology to potential partners in order to convert it into a high value-added product,” reveals Cunha.
The secret of the process

The CVD nanotube production processes take place inside a tube furnace into which gas containing carbon and catalytic nanoparticles are inserted. Subjected to high temperatures, the gas decomposes, and the carbon atoms deposit on top and around the nanoparticles, forming tubes (the nanotubes). The nanoparticles can be prepared in the same furnace used for nanotube growth.
The secret of the method developed by the Minas Gerais team lies precisely in the preparation of the catalytic nanoparticles. In broad lines, it is a matter of preparing a powder containing iron (Fe) and cobalt (Co) on aluminum flakes (material that had never before been mentioned in the scientific literature as a support for the growth of nanoparticles). The mixture is then subjected to temperatures of 350 to 650 °C for 4 hours, in an atmosphere similar to the air we breathe. This process, known as calcination, produces nanoparticles of iron and/or cobalt oxides. Then, the catalyst nanoparticles, still on the aluminum flakes, are introduced into the CVD furnace, whose internal temperature is brought to 730 °C. The ethylene gas (C2H4) is then introduced, which supplies the carbon so that the nanotubes grow perpendicular to the aluminum flakes.
Scientists observed an interesting advantage of using this new medium. During the calcination, a thin layer of aluminum oxide is formed on the surface of the aluminum that encapsulates the nanoparticles and prevents them from agglomerating or spreading. In addition, in the next step of the process, the aluminum oxide acts as a matrix of the nanotubes, driving their growth in the form of aligned bundles.
To test whether the calcination temperature of the nanoparticles would influence their performance as catalysts, the CTNano team carried out some experiments. The conclusion was that calcination at temperatures of 500-550 °C produces more mixed oxide nanoparticles (containing both iron and cobalt, of the CoFe2O4 formula) and produces better results in the production of nanotubes, both quantitatively (yield) and qualitative (diameter of the nanotubes).
“Unlike other methods described in the literature, which generally display low yield and are dependent on relatively expensive techniques (evaporation, sputtering) for the preparation of the catalyst, we describe in this paper a simple method to produce a catalyst in powder form, which can be used for continuous production of few-walled nanotubes using the chemical vapor deposition technique (CVD),” summarizes Thiago da Cunha.
CTNnano
The work was funded by the Brazilian agencies Fapemig (Minas Gerais State Research Foundation) and CNPq, as well as Petrobras. The work was carried out at CTNano, except for the microscopy images, conducted at the UFMG Microscopy Center.
CTNano emerged in 2010 based on the motivation to develop products, processes and services using carbon nanotubes and graphene, in order to meet industrial demands in line with the training of qualified human resources. The research realized in CTNano has already originated 26 patents and contributed to the development of more than 200 researchers in the area. According to Thiago da Cunha, CTNano will inaugurate, in 2018, its own headquarters with an area of approximately 3,000 m², located in the Technology Park of Belo Horizonte (BH-TEC).


