|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
[Paper: Nanocatalysts for hydrogen production from borohydride hydrolysis: graphene-derived thin films with Ag- and Ni-based nanoparticles. Leandro Hostert, Eduardo G. C. Neiva, Aldo J. G. Zarbin, Elisa S. Orth. J. Mater. Chem. A, 2018,6, 22226-22233. DOI 10.1039/C8TA05834B]
Graphene and nickel films, the best catalysts for hydrogen production
Thousands of vehicles powered by hydrogen gas already circulate in some regions of the world releasing only water through the exhaust pipes. As a fuel or source of energy, hydrogen is in fact an extremely clean (does not generate harmful emissions) and efficient option (it can produce more energy than any other fuel). However, pure hydrogen does not exist in nature on Earth. It needs to be produced, and most of the hydrogen-generating methods known to date have both economic and ecological drawbacks.
An alternative to these methods was recently presented by a team of researchers from the graduate program in Chemistry of the Brazilian Federal University of Paraná (UFPR). These scientists have proposed a clean, efficient, simple and inexpensive method to produce hydrogen. The team developed new catalysts (compounds that modify the speed of a chemical reaction without being consumed during the reaction), made of graphene and metal nanoparticles, which made hydrogen production feasible through the hydrolysis of borohydride – a chemical reaction still little used in hydrogen generation, notwithstanding its enormous potential as it is clean and very simple.
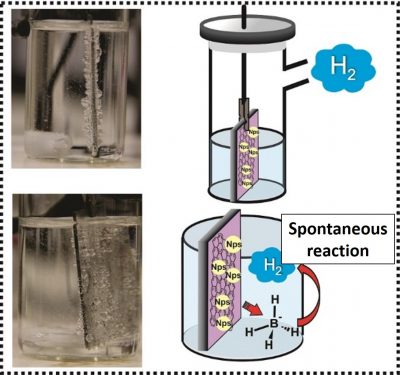
In this reaction, which is performed at room temperature, sodium borohydride (NaBH4) molecules, spontaneously react with water molecules generating hydrogen (H2) molecules. The process takes place in only one step, and is performed with catalyst materials, which accelerate the reaction rate.
“The main contribution of this work is the possibility of H2 generation through thin films of graphene nanocomposites,” says Professor Elisa Souza Orth, corresponding author of an article on the work, recently published in the Journal of Materials Chemistry A (impact factor = 9,931). “Nanocomposites of carbon-based materials and metallic nanoparticles have shown many promising applications and we have shown that for the less exploited borohydride hydrolysis they could also be used efficiently,” she adds.
Among the thin film catalysts produced by the UFPR team, the ones that presented better performance were those of reduced graphene oxide with nickel nanoparticles (rGO/Ni). In fact, this nanocomposite, produced with a relatively inexpensive metal, performed better than most of the catalysts previously reported in the scientific literature, including those prepared with noble metals, which cost much more.
In general, this means that small amounts of rGO/Ni (some tens of mg) generated large volumes of hydrogen (400 ml) in a short time (5 hours).
In addition, the films developed by the Brazilian team presented another important characteristic for a catalyst: they can be easily removed from the reaction vessel, washed and dried without damage, thus allowing their reuse. “In this work, we were able to reuse the same nanocatalyst in 10 consecutive cycles, without losing activity,” says Professor Orth.

These results were made possible by combining competencies in the production of carbon nanomaterials from the Materials Chemistry Group, coordinated by Professor Aldo José Gorgatti Zarbin, with expertise in catalysis processes of the Catalysis and Kinetics Group, led by Professor Orth. These two UFPR groups have a history of collaboration in the application of carbon materials; initially, in the study of pesticides and, currently, in the development of multifunctional materials with extraordinary catalytic activity.
In addition to the development of catalysts and their application in hydrogen production, the work published in the Journal of Materials Chemistry A included an analysis of the various ways of measuring the catalytic activity of a material. The authors were able to standardize criteria and compare several results obtained in the laboratory and found in the scientific literature. “We have developed a kinetic study that complements the discussion of these complex reactions and can help guide us to a more concise understanding of catalytic activity,” explains Elisa Orth.
The research was carried out under the doctoral program in progress of Leandro Hostert, guided by Professor Orth, and was funded by Brazilian agencies CNPq, CAPES, Araucária Foundation, INCT Nanocarbono, as well as L’Oréal-UNESCO-ABC through the Award for Women in Science (2015 ) and International Rising Talents (2016) received by Elisa Orth.