[Paper: Hybrid tantalum oxide nanoparticles from the hydrolysis of imidazolium tantalate ionic liquids: efficient catalysts for hydrogen generation from ethanol/water solutions. Virgínia S. Souza, Jackson D. Scholten, Daniel E. Weibel, Dario Eberhardt, Daniel L. Baptista, Sérgio R. Teixeira and Jairton Dupont. J. Mater. Chem. A, 2016, 4, 7469-7475. DOI: 10.1039/C6TA02114J.]
Super efficient nanoparticles to catalyze production of hydrogen, an alternative fuel.
While some automobiles which use hydrogen fuel are entering the market, scientists from around the world are still trying to find cleaner, more sustainable, safer and cost-effective ways to generate and store hydrogen. In fact, even though it is the most abundant element in the universe and found in the water and in numerous other compounds, hydrogen cannot actually be found in its pure form on our planet. It must therefore be obtained from other chemical compounds.
One of the best methods to produce hydrogen, from ecological and economical points of view, is water splitting. This technique consists of separating water molecules into its two primary elements, generating hydrogen (H2) and oxygen (O2) gases. This separation can be achieved through the use of the abundant solar energy, at room temperature. However, in practice, for sunlight to split one water molecule, it requires nanoparticles made of semiconducting materials to act as catalysts, or more specifically, as photocatalysts.
In a study fully carried out in Brazil, a team of scientists developed a new simple and efficient method to produce tantalum oxide nanoparticles (Ta2O5) with outstanding performance catalysts for hydrogen generation. The research was reported in a paper recently published in the Journal of Materials Chemistry A (impact factor: 8.262).

This study was funded by the Brazilian research agencies CAPES and CNPq, as the doctoral research of Virgínia Serra Souza at the Chemistry Institute of the Federal University of Rio Grande do Sul (IQ-UFRGS), under the guidance of Professor Jairton Dupont.
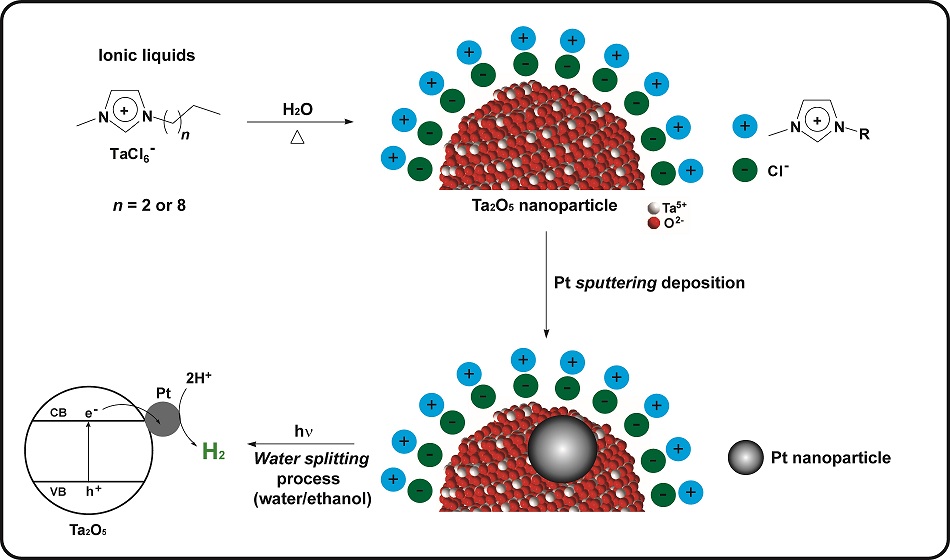
“The idea for this research came when we were looking for an alternative and efficient route for the synthesis of Ta2O5 nanoparticles, and after some experiments we decided to test the possibility of using ionic liquids as stabilizing sources and agents of the nanomaterials”, says Professor Jackson Damiani Scholten, who is one of the corresponding authors of the paper and member of the research group of IQ-UFRGS. This group has extensive experience in the study and development of ionic liquids (salts which are in liquid state at room temperature). Due to their physicochemical properties, ionic liquids can be used in the preparation of nanoparticles as stabilizers to keep the particles in the nanometric range.
Souza, Scholten and Dupont prepared two types of ionic liquids containing tantalum and create the conditions for the hydrolysis reaction (breaking the chemical bonds of a compound by the addition of water). The elements resulting from the hydrolysis, from the water and the ionic liquid, recombine to form tantalum oxide nanoparticles.
The team realized it had produced tantalum oxide nanoparticles ranging between 1.5 and 22 nm, the smaller ones had been generated from one of the ionic liquids and the larger ones from the other. With the assistance of Professor Daniel E. Weibel, also from IQ-UFRGS, they studied the surface composition of the nanoparticles. These scientists proposed that the nanoparticles obtained were hybrid: remains of ionic liquid were observed around the tantalum oxide.
To see how the nanoparticles behaved as catalysts in the separation of water molecules to generate hydrogen, the team conducted photocatalytic tests at the facilities of the Institute of Physics – UFRGS, provided by Professor Sérgio R. Teixeira. The tests were carried out in a solution that besides water contained ethanol – a compound that helps to increase the hydrogen production rate.
“We were delighted that the Ta2O5 nanoparticles showed one of the best results ever published for the production of H2 from a water/ethanol solution”, recalls Professor Scholten. In the article, this exceptional result was attributed to the presence of ionic liquid in the nanoparticles. “We believe that the residual ionic liquid enhances the formation of a hydrophilic regions on the surface of Ta2O5, favoring the approximation of polar molecules (water and ethanol)”, explains Scholten. To be certain about this, the scientists removed the ionic liquid from the nanoparticles by heat treatment and confirmed their very low photocatalytic activity.
In another stage of the research, Dario Eberhardt, then professor at the University of Caxias do Sul (UCS), collaborated with the team in the deposition of roughly 1 nm platinum nanoparticles on the surface of the hybrid tantalum oxide nanoparticles by the sputtering technique, carried out at IF-UFRGS. Professor Daniel L. Baptista, of IF-UFRGS, helped to characterize the material. In the tests, the performance of the tantalum oxide nanoparticles with photocatalytic ionic liquid was even better with the addition of platinum.
This work, carried out in southern Brazil, presented a new method to produce super-efficient catalysts for hydrogen production, a promising alternative fuel from water and ethanol, two renewable and abundant resources.