[Paper: Conversion of “Waste Plastic” into Photocatalytic Nanofoams for Environmental Remediation. Geovania C. de Assis, Euzébio Skovroinski, Valderi D. Leite, Marcelo O. Rodrigues, André Galembeck, Mary C.F. Alves, Julian Eastoe, and Rodrigo J. de Oliveira. ACS Appl. Mater. Interfaces, 2018, 10 (9), pp 8077–8085. DOI: 10.1021/acsami.7b19834].
Polystyrene, from pollutant to environmental remediator
A team composed of seven researchers from Brazil and one from the United Kingdom used polystyrene waste, which is a potential environmental polluter, to produce a material that works as an environmental remediator by degrading toxic compounds in water bodies and waterways. Therefore, the research makes a contribution to two disturbing environmental problems: on the one hand, the presence of large amounts of plastic waste on the planet; on the other hand, pollution or contamination with toxic substances in aquatic ecosystems.
The research was reported in a paper recently published in the journal Applied Materials & Interfaces (impact factor = 7,504).
We know that polystyrene is used to make disposable cups and cutlery, yogurt pots, combs, hangers, organizer boxes and many other utilities, in addition to being the main component of the well-known isopor®. “We present an alternative to reuse one of society’s most demanded plastics,” says Professor Rodrigo José de Oliveira, from Paraíba State University (UEPB), corresponding author of the paper.
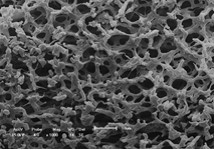
The material developed by the scientific team consists of a porous polymer matrix impregnated with nanoparticles of tin dioxide (SnO2). In this composite material, while the nanoparticles are largely responsible for degrading the toxic compounds (dyes) by a photocatalytic process, the polymer matrix, which is produced from polystyrene waste, creates an environment favorable to photocatalytic activity and supports the nanoparticles, allowing them to be easily removed from the waters, and reused in new processes of environmental remediation.
The authors of the article called the new composite material “nanofoam”. Although they have a few centimeters in diameter and pores of a few micrometers, the foams have received the prefix “nano” because their photocatalytic properties are due to the presence of nanometric-sized tin dioxide (spherical nanoparticles of about 20 nm). “Nanomaterials are those that present new properties due to a distinct physics that appears in this size scale,” recalls Oliveira.

To obtain composite foams, the team used low cost equipment and procedures based on well-known physicochemical properties. The preparation process is described in broad lines as follows. First, small pieces of polystyrene waste are dissolved in cyclohexane solvent, and the tin dioxide nanoparticles, which here were manufactured by the team, are added to the solution. This part of the process is carried out above the so-called “theta temperature (θ)” of the cyclohexane and polystyrene solution, which is about 36 °C, because below this temperature the solution undergoes a phase separation. Second, the preparation is carried out for 10 minutes at a temperature of 10 °C. As a consequence of this temperature decrease, some phenomena take place. The solution separates into two phases, one rich in polystyrene and the other rich in cyclohexane, and the solvent freezes. At the end of the cooling process, the phases are distributed in such a way that they form a polystyrene structure with holes filled with frozen cyclohexane. In order to remove the solvent from the foams, a lyophilization process is applied, whereby the cyclohexane undergoes sublimation. As a final result, a porous solid is obtained that the authors of the article called nanofoam.
“We have shown that a dense polystyrene waste can be readily converted into a porous polymer matrix, which is desirable for making new materials, that is, a noble end to pollution that has mobilized governments from industrialized countries,” says Professor Oliveira.
 Finally, the scientific team evaluated the efficiency of the new material as an environmental remediator by testing the ability of nanofoams to degrade a magenta-like dye called rhodamine B. This compound, which is used as a marker in health, research, agriculture areas, is toxic to the reproductive and neurological system, and in some studies it has been pointed out as a carcinogenic agent.
Finally, the scientific team evaluated the efficiency of the new material as an environmental remediator by testing the ability of nanofoams to degrade a magenta-like dye called rhodamine B. This compound, which is used as a marker in health, research, agriculture areas, is toxic to the reproductive and neurological system, and in some studies it has been pointed out as a carcinogenic agent.
The nanofoams of Professor Oliveira and his collaborators managed to degrade 98.2% of rhodamine B – a result superior to those obtained with photocatalytic nanoparticles outside the polystyrene matrix. In addition, the nanofoams demonstrated very good performance when reused: they degraded more than 96% of rhodamine B in the first four cycles. “It is desirable to use a matrix because it facilitates the final recovery of the photocatalyst, since the foam is easily removed from the medium using steel tweezers, in addition to an increase in the surface area due to higher oxide dispersion in the matrix,” says Oliveira.
History of the work
Rodrigo de Oliveira was involved in his doctorate when, in 2011, he had the idea of obtaining new catalytic materials, taking advantage of the characteristic of some solutions in which their phases are separated by the effect of temperature (known as “thermally induced phase separation,” TIPS). Oliveira was in an internship abroad (the so-called “doctoral sandwich”) in the group of Professor Julian Eastoe at the University of Bristol. In this group, Oliveira had found the double possibility of working with surfactants, the subject of his doctoral research, and also improving his command of the English language. “In Bristol, Julian presented me with a paper he had published decades ago on the use of TIPS to study microemulsions and the formation of calcium carbonate foams,” Oliveira recalls. By the end of the internship, the Ph.D. work had developed a surfactant foam decorated with gold nanoparticles. In England, in addition to this work in Bristol, Oliveira made contact with a renowned group at Cardiff University dedicated to catalysis research, led by Professor Graham Hutchings. “The possibility of obtaining new catalytic materials using TIPS was cogitated,” recalls Oliveira.
In 2012, Oliveira obtained a doctor’s degree in Chemistry from the Federal University of Pernambuco (UFPE). Shortly after, after qualifying in the selection process of UEPB, he became a professor of that institution. The young researcher then saw the opportunity to realize the idea he had envisaged in England. In order to carry it out, he had to venture into a new research line, different from what he had pursued during his undergraduate, master’s and doctoral works, but he was already experienced with this. In fact, during his undergraduate, master’s and doctorate courses in Chemistry, all of them at UFPE under the guidance of Professor André Galembeck, he had addressed three very different research topics. “André has always been open to proposals and ideas, including those that differed from the group’s research history,” declares Oliveira.
There was no lack of courage, but Oliveira had just arrived at UEPB and had no infrastructure available, nor the financial resources to build it. “From 2012 to now, our struggle has been to establish a research group in physical chemistry of materials, and one of the focused lines is the use of TIPS to make materials from plastic waste,” he says. In the case of nanofoams, the researcher was able to develop the work within Geovânia Cordeiro de Assis’s master’s thesis, defended in 2016 and supervised by Oliveira together with professor Mary Cristina Ferreira Alves (co-supervisor). For the preparation of the nanofoams, the team used simple and inexpensive equipment (“a $ 20 refrigeration plate purchased from China’s import website and a vacuum pump”). For the characterizations, which necessarily require more expensive equipment, Oliveira had the collaboration of colleagues from UFPE, University of Brasilia and University of Bristol.
Other materials with environmental applications will be generated in the city of Campina Grande, in the laboratory of Professor Oliveira, using various types of plastics, including those with a more complex composition such as styrofoam®, which is composed of expanded polystyrene and other chemical components. In addition to developing materials to contribute to the remediation of ecosystems, the group is using them for more fundamental studies, which could generate nanomaterials with sophisticated structures.
“Unfortunately, we are constantly faced with the lack of characterization equipment, and nowadays neither the collaborators with the best of intentions have the resources to help us as before,” Oliveira laments. “I realize there are quality human resources in our institution; however, more investments in infrastructure are fundamental to maintain the quality of the work, training of human resources and internalization of state of art science,” he adds.



