[Paper: Decreasing Nanocrystal Structural Disorder by Ligand Exchange: An Experimental and Theoretical Analysis. Gabriel R. Schleder, Gustavo M. Azevedo, Içamira C. Nogueira, Querem H. F. Rebelo, Jefferson Bettini, Adalberto Fazzio, Edson R. Leite. J. Phys. Chem. Lett. 2019 10 1471-1476. https://doi.org/10.1021/acs.jpclett.9b00439]
Disclosing structural disorder in nanomaterials
It is known that it is very important to know and control the structure of a material (how its atoms are arranged in three-dimensional space) as it is largely responsible for the properties of the material and therefore for its applications. For example: regions of disorder in crystalline materials (whose atoms, ideally, are ordered in regular patterns) change some expected behaviors for these materials. Unfortunately, knowing the structure of some materials in detail can be a difficult task – particularly when it comes to nanomaterials.
Concentrating various skills and experimental and theoretical resources, a Brazilian team developed a method to establish the degree and location of disorder in the structure of crystalline and non-crystalline nanomaterials, interfaces and surfaces. The method, which is based on the combination of an experimental technique (transmission electron microscopy), a data analysis method (pair distribution function) and computational simulations, is already available to the scientific community at the Brazilian National Nanotechnology Laboratory (LNNano), and should help develop better performing materials.
In addition to developing the technique, the team applied it in the study of structural disorder in nanocrystals, which are basic elements of nanotechnology and are used for example, in solar cells and electronic devices. Although by definition they have ordered structures, these crystals of nanometric dimension can exhibit, in practice, regions with structural disorder.
In order to carry out the study, the scientists produced faceted nanocrystals of about 3.2 nm in diameter, formed by a core of zirconium dioxide (ZrO2), inorganic material, and a shell made up of organic substances known as ligands, whose atoms form chemical bonds with atoms that are on the surface of the inorganic nucleus. Ligands have the important role of stabilizing the nanocrystals, thus preventing them from aggregating.
The team produced a first series of nanoparticles with ligands containing an aromatic ring and analyzed it using the developed method. The samples were then subjected to a process known as ligand exchange in which chemical reactions occur in the material in the presence of a solvent at a temperature above its boiling point. In these reactions, some connections break down and new connections occur. As a result of the ligand exchange, the team was able to produce nanoparticles with shells containing oleic acid, which were also analyzed using the developed method.
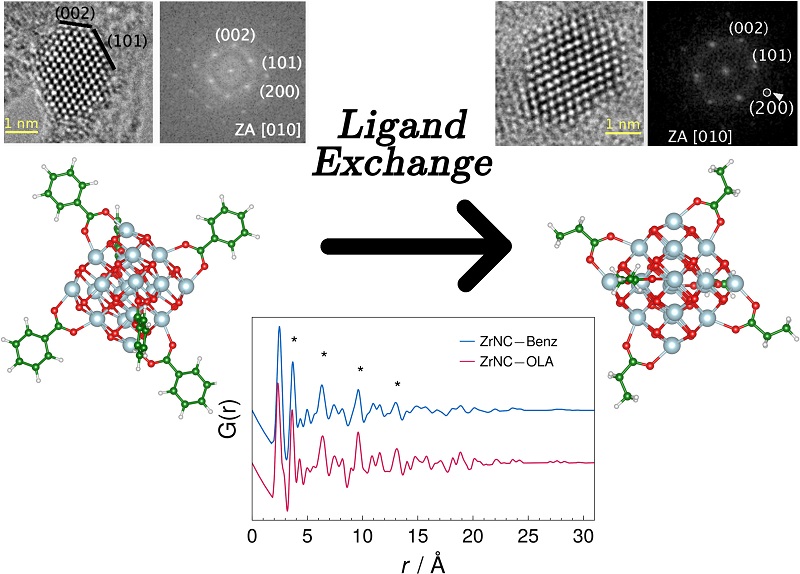
The scientists concluded that, unlike the ideal nanocrystal of zirconium dioxide, the two types of nanocrystals analyzed had a degree of structural disorder located on the surface of the nucleus. In addition, in the second group of nanoparticles, the disorder was significantly lower. The researchers interpreted this reduction as a result of the high temperature of the ligand exchange process, which altered the tensions of the network of atoms.
“In our work, we were able to directly assess the degree and location of disorder in the nanocrystals, which until then was not technically feasible,” says Gabriel Schleder, PhD candidate in the Graduate Program in Nanosciences and Advanced Materials of the Brazilian Federal University of the ABC (UFABC).
By better understanding structural disorder and its causes, the researchers were able to point out a way to control it. “Any property that significantly depends on surface-located structural disorder could be in principle controlled by this kind of ligand exchange process,” says Schleder. “Mechanical properties, photoluminescence, electronic transport and catalytic properties are some of them,” he adds.
The research was reported in a recently published article in The Journal of Physical Chemistry Letters (impact factor = 8,709).
Overcoming the challenge through collaborations
The initial idea of the study appeared in a meeting held at the end of 2017 at the National Center for Research in Energy and Materials (CNPEM), located in the city of Campinas, São Paulo. At the meeting, a group of reserachers discussed the implementation in Sirius (the next Brazilian synchrotron light source) of a technique that allows locally analyzing structural issues such as disorder and defects, called pair distribution function (PDF). The technique describes the distances between pairs of atoms by means of a mathematical function. To apply it, the specialist generally uses the results of X-ray diffraction measurements – an experimental technique that provides information about the structure of materials. However, in order to implement the analysis by PDF, the X-ray beam focused on the sample must be of very high energy – higher than that provided by the current Brazilian synchrotron light source.
During the meeting at CNPEM, Professor Gustavo de Medeiros Azevedo, researcher at the National Laboratory of Synchrotron Light (LNLS), and Professor Edson Leite, LNNano’s scientific director, decided to begin applying PDF using electron diffraction results, a specialty of LNNano’s researcher Jefferson Bettini. The electron beams would be generated by the transmission electron microscope (TEM) of LNNano. In fact, this instrument allows the control of the electron beam so that it focuses a small area of the sample, allowing the desired local analysis of the structure. Besides that, when switching from the “diffraction mode” to the “image mode”, the microscope would made possible to choose precisely the area of the sample to be analyzed.
The development team also involved professors Içamira Costa Nogueira, from the Federal University of Amazonas (UFAM) and Querem Hapuque Felix Rebelo, from the Federal University of the West of Pará (UFOPA), who contributed with the synthesis of nanocrystals that would be studied and with the development of the analysis methodology.
During the development of the technique, another challenge had to be faced. To interpret the PDF results, it would be necessary to have a simulation of an ideal nanocrystal – a nanocrystal model without structural disorganization that could be used as a reference.
New skills were then incorporated into the team, which was then joined by Professor Adalberto Fazzio, director general of LNNano and leader of a UFABC research group dedicated to computational techniques applied to materials, and his doctoral student Gabriel Schleder. Based on the Density Functional Theory (DFT), a computational modeling method in the field of Quantum Physics, the researchers were able to simulate the ideal nanocrystal that served as the analysis model.
“Something very positive we perceived is that the main results arose from the process of interaction, discussion and exchange of information mainly between theory/computational simulation and experiments. Without this, we certainly would not have good final conclusions,” says Schleder.



