|
||||||||||||||||||||||||||||||||||
To unsubscribe, click here
|
||||||||||||||||||||||||||||||||||
[Paper: Mean-field and linear regime approach to magnetic hyperthermia of core-shell nanoparticles: can tiny nanostructures fight cancer? Marcus S. Carrião, Andris F. Bakuzis. Nanoscale, 2016,8, 8363-8377. DOI: 10.1039/C5NR09093H]

Hyperthermia, for cancer treatment, is induced by increasing the temperature to activate tumor cell death. This high temperature can be created by introducing nanoparticles into the tumor that function as heaters, and after their function is completed they are eliminated by the body. Magnetic nanoparticles may be used in these treatments because they have the ability to generate heat when subjected to alternating magnetic fields of specific intensity and frequency.
A work on nanomedicine (nanotechnology used in medicine) fully conducted at the Brazilian Federal University of Goiás (UFG) suggests a new strategy using hyperthermia for cancer treatment: using smaller magnetic nanoparticles than those normally used and composed of more than one material, which could provide several advantages to the patient. To reach this conclusion, the researchers developed an innovative theoretical method that paves the way for manufacturing magnetic nanoparticles optimized for hyperthermia. The study was reported in a paper published in the prestigious Nanoscale journal, signed by the doctoral student Marcus Carrião dos Santos and his supervisor Andris Figueiroa Bakuzis, professor at the Physics Institute of UFG.
Hyperthermia cancer treatment generally uses nanoparticles that are relatively large (20 nm size range) and homogeneous (from a single material), which are considered as the most effective to generate heat according to theoretical studies based on traditional methods. However, these “large” nanoparticles accumulate quickly in the liver and it may take several months or years for these particles to leave the body of the patient being treated. On the other hand, nanoparticles smaller than 10 nm are rapidly eliminated in the urine, reducing the possibilities of intoxication and thereby increasing the selection of materials that can be used to manufacture them.
The relationship between particle size and excretion route (liver or kidney) was a conclusion reached by Bakuzis and colleagues from evidence reported in the scientific literature and pre-clinical studies (in vivo) carried out within a multidisciplinary research network, coordinated by Bakuzis, and aimed at solving problems associated with the use of magnetic nanoparticles for cancer treatment.
In addition, smaller nanoparticles have better distribution and penetration in tumors, among other advantages in the context of cancer treatment.
Aware of these characteristics, Bakuzis and dos Santos investigated the possibility of manufacturing nanoparticles of less than 10 nm that could efficiently generate heat. An important insight came from an article published in 2011 in the Nature Nanotechnology journal (Nat. Nanotech. 6, 418 (2011)). Professor Bakuzis says that “in this article, the researchers concluded experimentally that certain heterogeneous (from different materials) core-shell structures of spinel ferrites warmed more efficiently than homogeneous particles”.
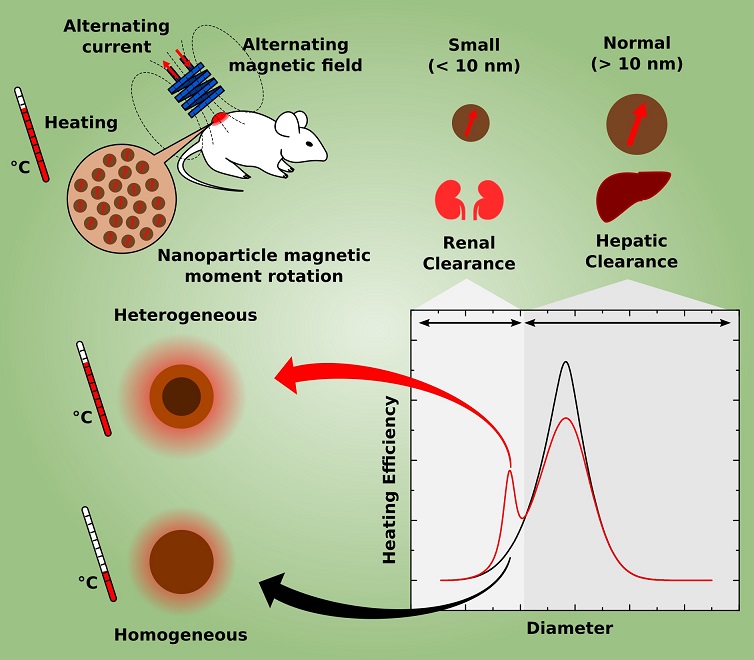
The pair of scientists then decided to theoretically investigate whether nanoparticles less than 10 nm formed by one material core and a shell of another material could efficiently generate heat and how to optimize them for this function. However, the conventional methods available for this modeling were not adequate. In fact, they considered the nanoparticle as a homogeneous entity, ignoring the fact that the surface atoms and the core atoms respond differently to the application of a magnetic field. This oversight became more significant in the study of particularly heterogeneous particles such as those they intended to study, the reason why the researchers from Goiás decided to develop a more suitable model for the study object. Bakuzis explains that “in the paper we presented the first analytical hyperthermia model of core-shell nanoparticles within the linear response and mean-field theory, and from these calculations we pointed out important materials properties to achieve efficient heat generation.”
The results obtained by the physicists and published in the paper may have a significant impact in a health issue that concerns humanity, cancer cure. “Our studies indicate that it is possible to develop small particles for cancer treatment that can be quickly eliminated from the body via the kidneys. In particular, by combining different materials in the nanostructure”, summarizes Bakuzis.
To work with impact on this interface theme, Bakuzis is always in contact with a pool of knowledge of various areas. In addition to leading the multidisciplinary nanomedicine network that includes researchers with backgrounds in tumor biology, genetics, physiology, pharmacy, veterinary medicine, biophysics, physics, medical physics and chemistry, the professor and his group actively participate in scientific events that bring together many different professionals, including doctors with various specializations already using hyperthermia in humans for cancer treatment. “These scientific contacts are fundamental in interface areas such as the one our group works with,” concludes Bakuzis.
The research that led to the paper in the Nanoscale journal received funding from the Brazilian National Scientific and Technological Development Council (CNPq) and from the Research Foundation of the State of Goiás (FAPEG) and was carried out as part of the doctoral work of Marcus Carrião dos Santos.