[Paper: Highly Controlled Diffusion Drug Release from Ureasil–Poly(ethylene oxide)–Na+–Montmorillonite Hybrid Hydrogel Nanocomposites. ACS Appl. Mater. Interfaces, 2018, 10 (22), pp 19059–19068. DOI: 10.1021/acsami.8b04559]
Clay Labyrinth in Hydrogel Matrix for Controlled Drug Release
By combining a clay and a polymer gel at the nanoscale, a brazilian scientific team with members of the São Paulo State University (UNESP) and the University of Franca (UNIFRAN) developed a new material that can carry drugs and release them in a gradual and controlled manner.
The team tested in vitro – that is, in the laboratory, in containers that simulate the biological conditions – the performance of the material in the release of sodium diclofenac. This drug is an anti-inflammatory, given orally or by injection, widely used to relieve swelling and pain from, for example, arthritis, rheumatism, muscle injuries, surgeries or gout.
The material developed is a nanocomposite that includes polymeric hydrogel, clay and the drug. The hydrogel (gel that absorbs water amounts higher than normal without dissolving) is composed of an organic-inorganic hybrid material known as siloxane-polyether or ureasil. The clay is known as montmorillonite, and is present in the nanocomposite in the form of nanometric lamellae homogeneously dispersed in the hydrogel. The diclofenac sodium, which appears encapsulated within the nanocomposite, is incorporated into the material during its preparation, as if it were another “ingredient”.
The nanocomposite was obtained by the São Paulo team through the sol-gel process. This preparation method is based on a series of chemical reactions with the transformation of a “sol” (liquid with nanometric particles in suspension) into a gel (rigid three-dimensional network with interstices in which the liquid remains immobilized).
In this nanocomposite the main function of the hydrogel, which is hydrophilic, is absorbing water from the external environment and storing it in its interstices. In this aqueous environment, the drug molecules disperse due to the physical diffusion process until they cross the pores of the hydrogel and exit into the external environment, in this case the human body if the material were being used to release drugs into real patients.
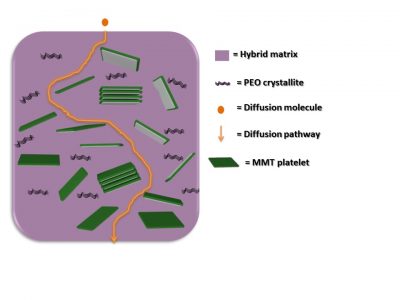 The main novelty of the material is the use of clay, which is impermeable, to control how the drug is released. In fact, in the material developed by the São Paulo team, the nanometric clay lamellae acted as a physical barrier to the passage of the molecules of water and drug.
The main novelty of the material is the use of clay, which is impermeable, to control how the drug is released. In fact, in the material developed by the São Paulo team, the nanometric clay lamellae acted as a physical barrier to the passage of the molecules of water and drug.
As shown in the image below, the lamella set formed a real labyrinth that slowed the movement of these molecules, determining a specific rhythm to water absorption and the release of diclofenac sodium.
“The main contribution of this work was to develop a barrier system based on an organic-inorganic hybrid material containing polymer-clay for the fine control of the diclofenac sodium release,” says Eduardo Ferreira Molina, corresponding author of an article on the subject, recently published in the journal ACS Applied Materials & Interfaces. Molina is currently a professor at the University of Franca (SP).
In the work reported in this journal, the authors prepared a series of samples of the nanocomposite using different proportions of montmorillonite clay, as well as samples of the clayless hydrogel. The scientists used different characterization techniques to analyze the structure of the nanocomposites and their phases (hydrogel and clay) and also to study water absorption and release of the drug in the material. The team was able thus to verify that the presence of the clay was essential to control the way the drug was released. By adjusting the clay percentage used in nanocomposite preparation, the researchers were able to prevent the early release of a large dose of sodium diclofenac (a common problem in drug delivery systems). They also succeeded in releasing it slowly and at a steady and predictable rate.
The results of this work may constitute a first step towards the use of this nanocomposite as a drug release system for prolonged treatments of arthritis, migraine, postoperative pain and etc. With a system like this, medication could be released gradually at the most appropriate doses and rates, keeping the ideal concentration of the drug in the bloodstream.

The work, which received funding from the Brazilian federal agencies CAPES and CNPq and the São Paulo State agency FAPESP, was carried out at the Chemistry Institute of UNESP, in the city of Araraquara, with the exception of small-angle X-ray scattering (SAXS) measurements, performed at the Brazilian Synchrotron Light Laboratory (LNLS), in the city of Campinas.
The research was developed between 2010 and 2014 in the doctorate in Chemistry of Celso Ricardo Nogueira Jesus, under the supervision of Professor Celso Valentim Santilli (UNESP) and Professor Sandra Helena Pulcinelli (UNESP). The idea, previously unpublished, of developing these nanocomposites to function as barriers to controlled drug release arose at the beginning of the doctoral research of Nogueira Jesus. The theme brought together themes developed in two other postgraduate works. On the one hand, Eduardo Molina’s doctoral research, guided by Professor Santilli, on siloxane-polyether for controlled release of drugs. In 2010, this work was in the final phase. And on the other hand, Márcia Hikosaka’s master’s work, guided by Professor Pulcinelli and completed a few years ago, on the preparation of nanocomposites with polymers and montmorillonite clay.



