[Paper: Effect of the incorporation of poly(ethylene oxide) copolymer on the stability of perovskite solar cells. Jeann Carlos da Silva, Francineide Lopes de Araújo, Rodrigo Szostak, Paulo Ernesto Marchezi, Raphael Fernando Moral, Jilian Nei de Freitas and Ana Flávia Nogueira. J. Mater. Chem. C, 2020,8, 9697-9706].
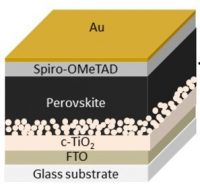
Thanks to the contributions of research groups from different countries, perovskite-based solar cells have quickly become competitive in terms of energy conversion efficiency – the percentage of solar energy that is converted into electrical energy – reaching values above 25%. Unfortunately, the good efficiency achieved for these solar cells does not remain throughout their use, mainly because of the instability of their active layer. Composed of materials from the perovskite family, this layer of the sandwich-like solar cell is responsible for absorbing light. Due to moisture, as well as light itself, perovskite degrades and threatens the life cycle of a solar cell.
The problem has been the focus of many researchers in the area, among them, those from the Laboratory of Nanotechnology and Solar Energy (LNES) at Unicamp (Brazil), led by Professor Ana Flávia Nogueira. In recently reported research in the Journal of Materials Chemistry C (impact factor 7.059), LNES members were able to produce more stable perovskite films which allowed manufacturing solar cells with lower efficiency losses over time.
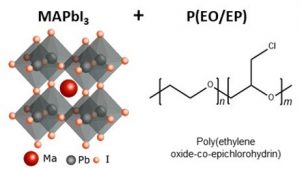
The strategy adopted was to add to the perovskite a compound that gives it stability without affecting its crystalline structure, from which important properties emerge for solar cell performance. The chosen additive, a copolymer (polymer formed by two different monomers), was added in different concentrations to a solution of lead iodide and methylammonium iodide, which, when crystallized, formed a modified and more stable perovskite film.
The researchers used the spin coating technique to prepare filmes of pure perovskite and “additivated” perovskite. In a material degradation test, the authors exposed the samples to ambient light and humidity for nine days and observed their degradation, which was visible to the naked eye by the yellowing of the films, whose original color is almost black. In the samples with additive, the degradation was delayed by a few days when compared to the pure perovskite samples.
Another test carried out by the team showed the films’ ability to regenerate after an initial degradation caused by exposure to a humidifier. The samples with the additive not only degraded less, but also spontaneously regenerated, almost entirely, thirty seconds after removing the moisture source – a phenomenon known as healing – as can be seen in this video.
“This work demonstrated that incorporating a copolymer based on poly(ethylene oxide) to the perovskite layer can delay and, in some cases, even reverse the degradation process of the film with relation to moisture and lighting,” summarizes Jeann Carlos da Silva, co-author of the article.
 To study in detail the structure and composition of the films, the authors used a series of characterization techniques, including an X-ray diffraction technique (in situ GWAXS), available at the Brazilian National Synchrotron Light Laboratory (LNLS), which allowed to monitor the manufacturing process of the films. Based on the set of characterization results, the authors were able to explain the mechanism that generates the protective effect in perovskite films with additives. According to them, the effect occurs mainly due to the interaction performed by the copolymer, through hydrogen bonds, with the methylammonium cation of the perovskite. In films without the additive, light and moisture cause part of the methylammonium to shift into the gas state and then leave the perovskite structure, generating the degradation, which is partially irreversible. In the films with the additive, the copolymer retains the methylammonium, which generates films that are more stable and have greater regenerative capacity.
To study in detail the structure and composition of the films, the authors used a series of characterization techniques, including an X-ray diffraction technique (in situ GWAXS), available at the Brazilian National Synchrotron Light Laboratory (LNLS), which allowed to monitor the manufacturing process of the films. Based on the set of characterization results, the authors were able to explain the mechanism that generates the protective effect in perovskite films with additives. According to them, the effect occurs mainly due to the interaction performed by the copolymer, through hydrogen bonds, with the methylammonium cation of the perovskite. In films without the additive, light and moisture cause part of the methylammonium to shift into the gas state and then leave the perovskite structure, generating the degradation, which is partially irreversible. In the films with the additive, the copolymer retains the methylammonium, which generates films that are more stable and have greater regenerative capacity.
“This study also allowed to investigate the crystallization dynamics of the perovskite containing the copolymer, as well as to understand the formation mechanisms of perovskite/copolymer in humidity and lighting conditions,” highlights Francineide Lopes de Araújo, co-author of the article. “In addition, through characterization techniques such as in situ X-ray diffraction, the study explores an important area in order to understand the material, offering an important contribution to the scientific community and opening new investigation perspectives for the application of polymers in the process of forming and manufacturing perovskite solar cells,” she adds.
Finally, the scientific team manufactured solar cells using perovskite films with and without additives as active layer, and compared their energy conversion efficiency. Initially, the presence of the copolymer decreased the efficiency of the devices, since, as it is an insulating material, it impairs the transfer of electrical charges. However, in the stability tests, when the devices were exposed to humidity and light for twenty days, the perovskite cells with additives performed better.
In numbers: while pure perovskite solar cells started at 17% efficiency and maintained 47% of that value at the end of the test, perovskite devices containing 1.5 mg mL-1% copolymer had an initial efficiency of around 15 %, but retained 68% of efficiency after the 20 days of testing.
“Unfortunately, the problem of stability of perovskite solar cells could not be definitively solved through this research, however, an important way to protect the material was explored, mainly against aggressive exposure to moisture and light, which in the future can be combined with other protection mechanisms,” summarizes Jeann Carlos da Silva. “The research also reinforces the feasibility of incorporating extrinsic compounds to perovskite as protective agents,” he adds.
This study began at LNES in 2016, in the master’s research of Jeann Carlos da Silva, shortly after the development, in that same laboratory, of the first perovskite solar cell prototype in Brazil. The research was completed with the collaboration of the postdoctoral fellow Francineide Lopes de Araújo and other members and former members of the group, always under the guidance of Professor Ana Flávia.
The study was funded by Brazilian agencies FAPESP, CNPq and CAPES, and is the subject of the project “Perovskite Solar Cells for Artificial Photosynthesis” of the Center for Innovation on New Energies (CINE) with support from Shell and Fapesp.








