In research carried out in a number of Brazilian laboratories, a multidisciplinary scientific team developed a magnetic, luminescent nanomaterial capable of chemically binding to molecules of interest, such as drugs or proteins. This nanomaterial also showed low toxicity in tests with living organisms. With this set of characteristics, the new material can be seen as a multifunctional nanoplatform that is promising for the development of various applications, especially in the areas of biotechnology, health and environment. The study was reported in an article published in ACS Applied Nano Materials (American Chemical Society journal released in 2018), and featured on the cover of the June issue of the journal.
The properties of this nanoplatform derive from the presence of several compounds and elements with distinct properties: iron oxide (Fe3O4, known as magnetite) nanoparticles responsible for magnetism; lanthanide element ions (Gd3 +, Ce3 + and Tb3 +, known as rare earths) responsible for luminescence or light emission, and chitosan (biopolymer obtained from the crustacean exoskeleton), essential for providing chemical bonds of the nanoplatform surface to the external molecules of interest.
The nanoplatform was developed at the Brazilian National Nanotechnology Laboratory of the National Center for Energy and Materials Research (LNNano – CNPEM). The process used for its synthesis comprises a series of steps. Initially, the iron oxide nanoparticles that form the core of the nanoplatforms are synthesized and coated with silicon dioxide (SiO2). Then the luminescent elements and chitosan are incorporated into the nanoparticles forming an outer layer. The result is nanoplatforms of approximately 170 nm in diameter (on average), called Fe3O4@SiO2/GdOF:xCe3+,yTb3+.
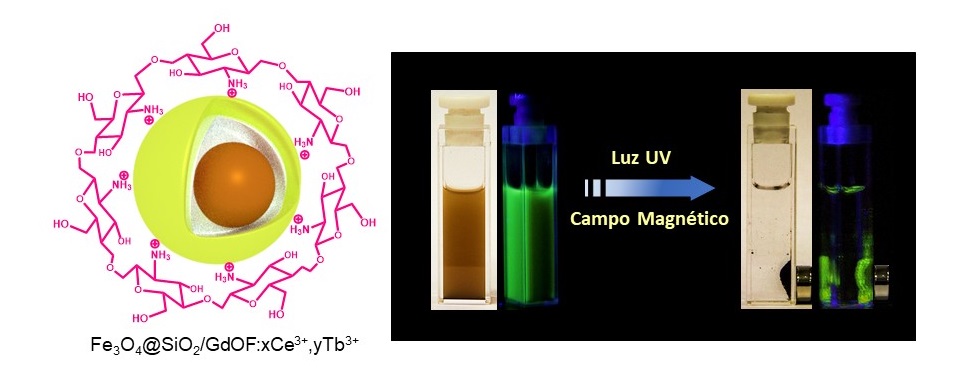
To study the magnetic and luminescent properties of the nanoplatform and to characterize its structure and morphology, research groups from the State University of Campinas (Unicamp) and the University of São Paulo (USP) participated in the study.
In addition, the main authors of the paper decided to evaluate the toxicity of nanoplatforms with relation to living organisms – a key step when thinking about health or environmental applications, and they decided to conduct a well-established in vivo test, in which zebrafish embryos (scientific name Danio rerio) are exposed to the material whose toxicity is to be evaluated. These freshwater fish, in fact, has a high genetic similarity to humans (about 70%) and at the same time is cheaper and easier to study than mice or rats, among other advantages.
In the toxicity test, a few dozen freshly fertilized zebrafish eggs were placed in aqueous medium containing the nanoplatforms at various concentrations. The embryos were examined at different development stages using an optical microscope to check for mortality, malformation, edema or changes in size. Tests included embryos with and without chorion (membrane that protects the embryo in the early stages of development). The test results carried out at LNNano showed that nanoplatforms, even at high concentrations (100 mg/L), have low toxicity for all embryo groups.
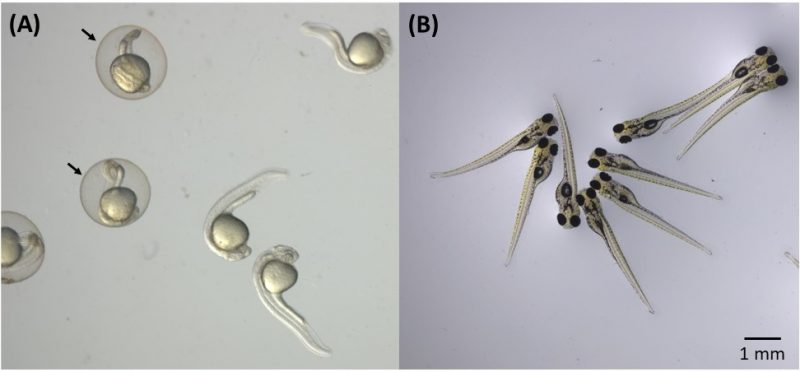
“This work brings an unprecedented contribution that involves evaluating the toxicity of hybrid nanomaterials using the zebrafish model, a promising alternative method in nanotoxicology, and the influence of the chorion,” says Diego Stéfani Teodoro Martinez, CNPEM researcher at LNNano and one of the corresponding authors of the article.
The embryos were also analyzed at the Brazilian National Synchrotron Light Laboratory (LNLS – CNPEM) to verify the distribution and concentration of nanoplatforms in the organism of the embryos. To do this, the scientists used the synchrotron light X-ray fluorescence microscopy (SXRF) technique, which can accurately map certain chemical elements in biological systems. This technique is available at one of the LNLS experimental stations, coordinated by the researcher Carlos Alberto Pérez, who is one of the corresponding authors of the article.
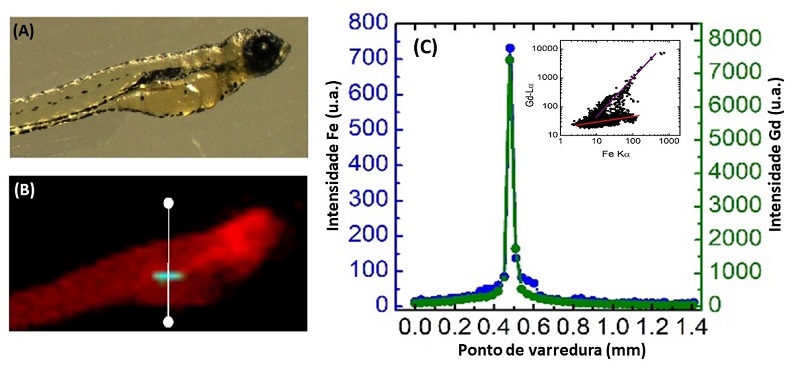
SXRF analysis showed that nanoplatforms had accumulated in the embryos as a function of exposure time, with higher concentrations in the gastrointestinal tract in the case of already developed mouth embryos – a result that may be significant, for example in the context of healthcare applications involving oral nanoplatform ingestion.
The study was carried out in the context of a postdoctoral project by fellow Latif Ullah Khan, also corresponding author of the article. The completion of the project, says Martinez, was made possible by the availability of skills and facilities at CNPEM’s multi-user laboratories. However, partnerships with other laboratories were also crucial, adds the CNPEM researcher. Professor Marcelo Knobel’s group performed the magnetometry studies at Unicamp. The groups of professors Hermi Felinto Brito and Magnus Gidlund carried out the luminescence and functionalization studies at USP. Finally, Professor Diego Muraca (Unicamp) and researcher Jefferson Bettini (CNPEM) contributed to the structural and morphological characterization using transmission electron microscopy techniques.
“This article was the result of integrating the experience of different Brazilian groups; an interdisciplinary study on the frontier of knowledge in nanobiotechnology and nanotoxicology,” says Martinez, adding that one of the main challenges of the work was integrating knowledge and techniques from different areas, such as Materials, Biology and Toxicology, a task that was coordinated by Martinez and Pérez.

The study received financial support from Brazilian agencies CAPES (including through the CAPES-CNPEM agreement), FAPESP and CNPq (including through INCT-Inomat); from the Brazilian Ministry of Science, Technology, Innovations and Communications (MCTIC) through SisNANO, and The World Academy of Sciences for advancement of science in developing countries (TWAS). The study also received financial support from the Brazil-China Nanotechnology Research and Innovation Center (CBC-Nano).
Applications: biotechnology, health and the environment
According to Martínez, the nanoplatform developed opens perspectives for applications in biotechnology, health and the environment, such as biological tissue and cell imaging systems, medical diagnostic kits, and environmental systems for pollutant detection and remediation
The applications would take advantage of the interesting set of nanoplatform properties. Because they are magnetic, using an external magnet, nanoplatforms could be directed and retained in a particular biological tissue or isolated from, for example, contaminated blood or water. In addition, the luminescence of the nanomaterial would allow visualizing the nanoplatforms within the biological tissues and cells of interest. Finally, the presence of chitosan would enable the chemical binding of drugs and other molecules that would serve for the diagnosis and/or treatment of diseases. “However, much study is still needed for real applications and commercialization of this nanoplatform, as it is a new material and needs to be tested on different models in the future,” says Martinez Martinez.
[Paper: Fe3O4@SiO2 Nanoparticles Concurrently Coated with Chitosan and GdOF:Ce3+,Tb3+ Luminophore for Bioimaging: Toxicity Evaluation in the Zebrafish Model. Latif U. Khan, Gabriela H. da Silva, Aline M. Z. de Medeiros, Zahid U. Khan, Magnus Gidlund, Hermi F. Brito, Oscar Moscoso-Londoño, Diego Muraca, Marcelo Knobel, Carlos A. Pérez, Diego Stéfani T. Martinez. ACS Appl. Nano Mater. 2019, 2,6, 3414-3425. https://doi.org/10.1021/acsanm.9b00339.]

