Two-dimensional materials, those whose thickness goes from an atom to a few nanometers, have unique properties related to their dimensionality and are protagonists in the development of nanotechnology and nanoengineering.
A team of scientists from five Brazilian institutions and one American institution took an important step in the development of the two-dimensional diamond version. This work on 2D diamond was reported in a paper published in Nature Communications (impact factor 12,124) with open access.
“Our work presented spectroscopic evidence of the formation of a two-dimensional diamond, which we named diamondene”, says Luiz Gustavo de Oliveira Lopes Cançado, professor at the Brazilian Federal University of Minas Gerais (UFMG) and corresponding author of the paper. In choosing the name of the new material, the scientists followed the tradition of using the suffix “ene” for two-dimensional materials, as with graphene, 2D version of the graphite.
 In fact, it was from the compression of graphene sheets that the diamondene was obtained by the team led by Professor Cançado. Initially, the team deposited two layers of graphene one on top of the other and transferred the graphene bilayer to a Teflon substrate, chosen for being chemically inert, preventing the formation of bonds with the graphene.
In fact, it was from the compression of graphene sheets that the diamondene was obtained by the team led by Professor Cançado. Initially, the team deposited two layers of graphene one on top of the other and transferred the graphene bilayer to a Teflon substrate, chosen for being chemically inert, preventing the formation of bonds with the graphene.
The sample of bi-layered graphene on Teflon was then subjected to high pressures and simultaneously analyzed by Raman spectroscopy at the Laboratory of Vibrational Spectroscopy and High Pressure of the Department of Physics of the Brazilian Federal University of Ceará (UFC). The experimental system used was a diamond anvil cell with a coupled Raman spectrometer. This equipment allows high pressure to be applied to small samples that are immersed in a pressure transmitting medium (in this case, water). The pressure is applied through two pieces of diamond (material chosen for being one of the hardest and resistant to compression), which compress the transmitting medium, which passes the pressure to the sample. At the same time, the spectrometer allows to monitor the changes that occur in the structure of the sample material against the different pressures applied. “In Raman spectroscopy, light behaves like a probe that measures vibrational states of the material,” explains Cançado. As a result of the probing, the spectrometer generates graphs (spectra), through which it is possible to identify the structure of the material being studied.
By analyzing the spectra, the team of scientists observed changes in the two-dimensional material that indicated the transition from a graphene structure to a diamond structure. The researchers were able to conclude that the diamondene was obtained at a pressure of 7 gigapascals (GPa), tens of thousands of times higher than the atmospheric pressure. “The evidence we present in this work is a signature in the vibrational spectrum obtained from a two-dimensional carbon material that indicates the presence of sp3 bonds, typical of the structure of the diamond,” says Professor Cançado.
To explain the formation of diamondene, the team used first principles calculations following the Density Functional Theory and Molecular Dynamics simulations. “These theoretical results guided the experiments and allowed us understanding the experimental results,” says Cançado.
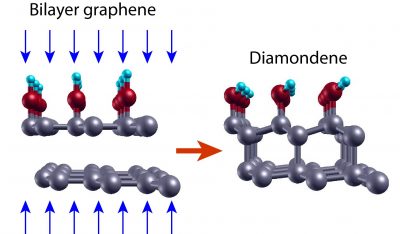
According to the theoretical results, when the bilayer graphene system on inert substrate with water as pressure transmitting medium is subjected to high pressures, the distances between the elements of the system decrease and new connections occur among them. “When applying this level of pressure on graphene, connections can change, going from the sp2 configuration to the sp3 configuration,” explains Professor Cançado. The carbon atoms in the upper graphene layer then establish covalent bonds with four neighboring atoms: the atoms of the lower layer and the chemical groups offered by water (OH- and H). The latter are fundamental to stabilize the structure. In the lower layer, in contact with the inert substrate, half of the carbon atoms are bound to only three neighboring atoms. “The pending connections give rise to a gap opening in the electronic structure, as well as polarized spin bands,” adds Cançado.
This feature makes diamondene a promising material for the development of spintronics (the emerging strain of electronics at the nanoscale in spin-bases electronics). According to Cançado, diamondene could also be used in quantum computing, microelectromechanical systems (MEMS), superconductivity, electrodes for electrochemistry-related technologies, DNA engineering substrates and biosensors – applications in which thin diamond films have already proven to have good performance.
However, there is still a long way to go before demonstrating the diamondene applications. Firstly, because the diamondene shown in the article dismantles under normal pressure conditions. To overcome this limitation, the group of Professor Cançado at UFMG is setting up an experimental system that will allow the application of much higher pressures to the samples in the order of 50 GPa and analyze them using Raman spectroscopy. “With this we intend to produce stable diamondene samples, which remain in this form even after having the pressure reduced to the level of ambient pressure,” says Cançado.
In addition, since Raman spectroscopy provides indirect evidence of the structure of the material, it will be necessary to perform direct measurements of the diamondene to know its structure in detail. “The most promising techniques in this case would be X-ray diffraction in synchrotron light sources or electron diffraction,” suggests Cançado. “The complicating factor in this experiment is the need to have the sample subjected to high pressures,” he adds.
The Brazilian history of diamondene
The idea of the 2D diamond formation originated in the doctoral research of Ana Paula Barboza, conducted under the guidance of Professor Bernardo Ruegger Almeida Neves and defended in 2012 in the Department of Physics of UFMG. In this work, Cançado says, atomic force microscopy (AFM) tips were used to apply high pressures on one, two and several layers of graphene. Indirect evidence of the formation of a two-dimensional diamond was obtained by means of electric force microscopy (EFM). The work showed the importance of the presence of two layers of graphene and water for the formation of the sp3 two-dimensional structure. The main results of the research were reported in the article Room-temperature compression induced diamondization of a few-layer graphene [Advanced Materials 23, 3014-3017 (2011)].

“The idea of measuring the Raman spectrum of graphene under high pressure conditions (using anvil diamond cells) came after Luiz Gustavo Pimenta Martins, an undergraduate student at the time, developed a very efficient method of transferring graphene to different substrates,” says Professor Cançado. This development was carried out during a visit to the laboratory of Professor Jing Kong at the Massachusetts Institute of Technology (MIT), after having won a grant for international mobility of the Formula Santander Award. During his master’s degree at the Physics Department of UFMG, carried out under the guidance of Professor Cançado and defended in 2015, Pimenta Martins carried out an extensive and systematic work to obtain Raman spectra of graphene samples subjected to high pressures. “There were many visits to UFC and much study until understanding the diamondene formation mechanisms,” explains Cançado.
The research reported in the Nature Communications paper was made possible by the collaborative work of several Brazilian research groups with recognized expertise in various subjects, as well as the participation of the MIT researcher in the sample preparations. Scientists from the physics departments of UFMG and UFC have contributed their recognized expertise in Raman spectroscopy applied to carbon nanomaterials and, in the case of UFC, in experiments under high pressure. Also participating in these experiments were researchers from the Brazilian Federal Institute of Education, Science and Technology of Ceará and the Brazilian Federal University of Piauí (UFPI). In addition, theoretical physicists from the Brazilian Federal University of Ouro Preto (UFOP) and UFMG performed calculations and computational simulations.
The research was funded by Brazilian federal agency CNPq, state agencies FAPEMIG and FUNCAP, Formula Santander Program and UFOP.
[Paper: Raman evidence for pressure-induced formation of diamondene. Luiz Gustavo Pimenta Martins, Matheus J. S. Matos, Alexandre R. Paschoal, Paulo T. C. Freire, Nadia F. Andrade, Acrísio L. Aguiar, Jing Kong, Bernardo R. A. Neves, Alan B. de Oliveira, Mário S.C. Mazzoni, Antonio G. Souza Filho, Luiz Gustavo Cançado. Nature Communications 8, Article number: 96 (2017). DOI:10.1038/s41467-017-00149-8. Disponível em: https://www.nature.com/articles/s41467-017-00149-8]
