|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
[A reversible, switchable pH-driven quaternary ammonium pillar[5]arene nanogate for mesoporous silica nanoparticles. Santos, ECS ; dos Santos, TC; Fernandes, TS; Jorge, FL; Nascimento, V; Madriaga, VGC ; Cordeiro, PS; Checca, NR; Da Costa, NM; Pinto, LFR; Celia Ronconi. J. Mater. Chem. B, 2020,8, 703-714. https://doi.org/10.1039/
A molecular machine to fight cancer
In 2016, the smallest man-made machines ever created, called molecular or nanomachines, gained visibility with the Nobel Prize in Chemistry. These nanometer-sized machines, whose components are molecules that perform controlled movements, could help humanity accomplish complex tasks at the molecular scale.
In the health area, one such task is to effectively fight cancer cells without damaging healthy tissues. It is known that nowadays one of the main problems of the most used therapies concerns the side effects on healthy tissues – a problem that has led many scientists to develop drug delivery systems that can take drugs directly to cancer cells without leaking.
At the Brazilian Federal Fluminense University (UFF), over the last ten years Professor Célia Machado Ronconi and her scientific team have been working on nanomachines for cancer treatment. In her postdoctoral research, carried out between 2003 and 2005, the scientist learned about molecular machines at the University of California, Los Angeles (UCLA), at one of the most qualified laboratories in the world working on this subject – the research group of Sir James Fraser Stoddart, who years later would be awarded the Nobel Prize mentioned at the beginning of this article, alongside with Jean-Pierre Sauvage and Bernard L. Feringa.
In a recently published paper in Journal of Materials Chemistry B, Professor Célia Ronconi, her team and collaborators, all from Brazilian institutions, presented a new nanomachine composed of a drug reservoir and a cap. The machine has an opening/closing lid mechanism that responds to changes in the acidity of the medium in which it is located. When the pH of the medium is similar to that of the blood of a healthy human being (physiological medium), the cap remains closed, preventing the drug from being released. When the pH is more acidic, a characteristic seen around cancer cells, the lid opens and the drug is released. In laboratory in vitro tests, the nanomachine loaded with a well-known chemotherapeutic drug proved to be more effective than the pure drug in eliminating breast cancer cells, destroying 92% of them in 48 hours.
With these characteristics, the nanomachine developed at UFF shows application potential in the delivery of chemotherapeutic drugs to cancer cells. “The results of this work were extremely promising,” says Professor Ronconi. “However, there is still much to be studied. The next steps of the work will be to test the nanomachine loaded with the drug in other breast cancer cell lines, as only one line (MCF-7) was tested. We will also test the toxicity of the device without the drug in healthy cells and, if the results are positive, in vivo studies will be carried out, using mices genetically altered to have a deficient immune system, ” adds Professor Ronconi.
Assembly and operation of the nanomachine
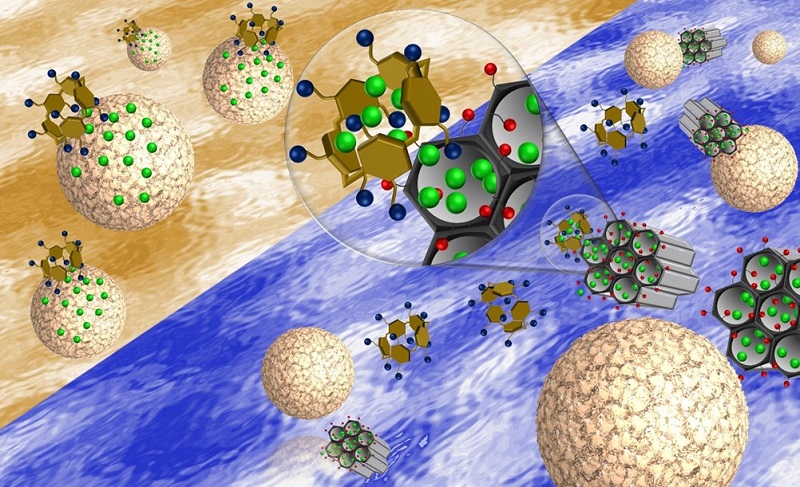
To achieve the reservoir function, the UFF group synthesized spherical mesoporous silica nanoparticles of about 85 nm in diameter. In addition to being biocompatible, this material has a unique internal honeycomb-like structure, with a set of nanochannels of up to 4 nm in diameter, in which the drug molecules can be stored. The nanoparticles were covered with carboxyl groups (- COOH) that improved the interaction of the reservoir with its cap. For the cap, the researchers chose pilararene, an artificial molecule made up of five aromatic rings, whose first synthesis dates back to 2008 in the scientific literature.
In the assembly and operation of the nanomachine, the electrostatic interactions of attraction controlled by the medium pH were the great allies of the scientific team at UFF. In fact, as confirmed by the researchers in their experiments, in a solution with a pH of 7.4, which represents the acidity of healthy blood, the carboxyl groups (-COOH) that cover the reservoir lose a proton forming carboxylate groups (-COO- ), negatively charged, which interact electrostatically with the positively charged cap. Thus, the electrostatic attraction brings the two parts of the nanomachine together until it prevents the drug from being released. By lowering the pH, that is, by making the solution more acidic, the carboxylate groups (-COO-) gain protons, neutralizing their charge. As a result, the electrostatic attraction between the cap and the reservoir breaks apart, the cap opens and the drug is released.
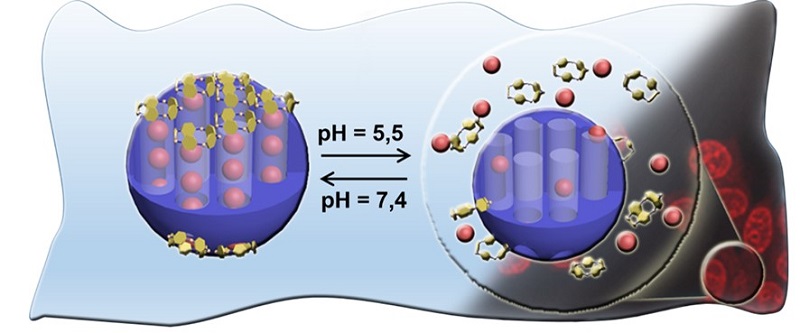
In the experiments carried out, the UFF group was able to partially release the chemotherapeutic drug (34%) at a pH of 5.5 (probably similar to that surrounding the cancer cells) and almost totally (91%) in a 2.0 acidity medium. All experiments were carried out at a temperature of 37 °C, similar to that of the human body.
History of work
Since 2009, when she became a professor at UFF and set up the Laboratory of Supramolecular Chemistry and Nanotechnology, Professor Célia Ronconi has been working in the different development phases of diverse nanomachines and drug transport and release systems, using chemical, magnetic and luminous stimulants. During Evelyn da Silva Santos’ doctorate, under the guidance of Ronconi, a nanomachine prototype was developed using material available on the market. However, new studies carried out after the defense of her doctorate work, in 2018, showed that the nanoparticles used as reservoirs formed clusters in the physiological environment (the solution that emulates blood in experiments). Thus, Professor Ronconi involved postdoctoral fellow Thiago Custódio dos Santos and doctoral student Tamires Soares Fernandes in the development of new material. “They continued the project and synthesized a material with excellent dispersion in the physiological environment, and the device was redone, as well as the drug release studies,” says professor Ronconi. The biological tests of the nanomachine were performed at INCA’s molecular carcinogenesis group, by researchers Luis Felipe Ribeiro Pinto and Nathália Meireles da Costa, and the technician Fernanda Jorge. The study also included the participation of the Brazilian Center for Research in Physics (CBPF) in the characterization of materials by microscopy techniques, carried out at the Multi-User Laboratory for Nanoscience and Nanotechnology (LABNANO). The research received funding from the Brazilian agencies CNPq, CAPES and FAPERJ.
