[Paper: Charge transfer effects on the chemical reactivity of PdxCu1−x nanoalloys. M. V. Castegnaro, A. Gorgeski, B. Balke, M. C. M. Alves and J. Morais. Nanoscale, 2016,8, 641-647. DOI: 10.1039/C5NR06685A]
Taming the reactivity of nanoalloys
When, in 2009, the Electron Spectroscopy Laboratory (LEe-) group of the Federal University of Rio Grande do Sul (UFRGS) decided to start developing in-house metal nanoparticles required for their studies, they came across some issues. Many synthesis methods reported in the literature did not provide the expected results when made in the Brazilian laboratory.

Strongly motivated by curiosity, as usual, says professor Jonder Morais, LEe- researcher, the group members were able, after much dedication, to develop new routes of synthesis that, in addition to being reproducible, are environment-friendly, efficient and cost-effective. “The first articles were published in international journals in 2013, initially with palladium (Pd), platinum (Pt) and silver (Ag) nanoparticles applied to the catalytic decomposition of nitric oxide. Subsequently, we published some works focused in “in situ” studies aimed at determining the mechanisms of formation and growth of monometallic nanoparticles. We have recently started reporting the results obtained with more complex systems, such as palladium and copper (Pd-Cu) nanoalloys,” states Professor Morais.
The latter group includes the results recently reported in an article published in the journal Nanoscale, whose main authors are Professor Jonder Morais and Marcus Vinicius Castegnaro, a physics doctoral student at UFRGS, advised by Morais. The research covered the entire process from the production of nanomaterials to the survey of their applications. “It was important to have dedicated students, willing to face the challenge of preparing accurately their own samples, and correlating the electronic and structural properties to understand the final properties in terms of chemical reactivity,” says Morais.
In the article published in Nanoscale, nanoparticles composed of palladium and copper alloys were produced by applying a simple method developed by the LEe- team. This process is carried out under mild conditions to the environment and health (aqueous, ambient temperature and pressure, and use of cheap and innocuous substances, such as ascorbic acid and sodium citrate). Several samples were synthesized by this route, containing three different amounts of palladium and copper atoms.
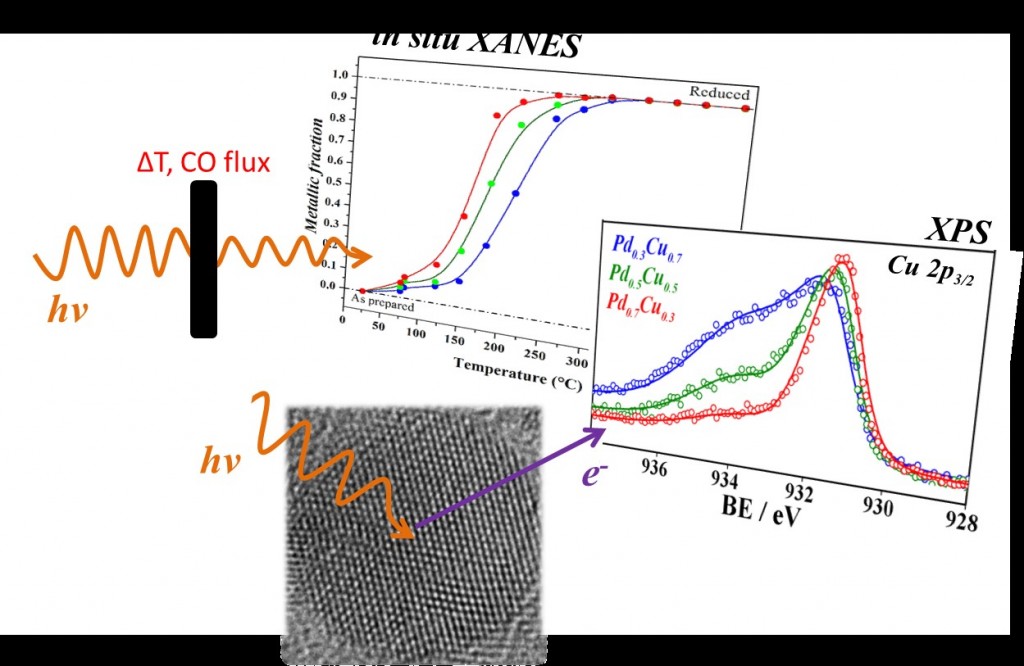
The synthesized nanoparticles have undergone a series of analyses conducted at UFRGS, in Porto Alegre (Rio Grande do Sul State), they traveled to Campinas (São Paulo State) for another series of analyses on equipment of the National Center for Research in Energy and Materials (CNPEM) and crossed the ocean to Johannes Gutenberg University, in Germany, for some additional measures. From characterization, the authors concluded that the nanoparticles were approximately 4 nm in size and were highly crystalline, among other characteristics. In addition, through experiments conducted by the XANES in situ technique, the team of scientists exposed the nanoparticles to carbon monoxide (CO) at 450 ° C and surveyed the reactivity of the nanoalloys, i.e., their ability to react chemically.
After studying the results of the characterization, the authors of the article were able to conclude that the alloy composition affects the ability of nanoalloys to reduce (gain electrons) and to oxidize (lose electrons). In fact, the greater the amount of palladium, the easier the reduction, and the harder the oxidation.
“The published results, obtained by the association of several experimental techniques are relevant to an understanding of the origin of high catalytic reactivity of palladium and copper (Pd-Cu) nanoalloys, as well as to elucidating similar behavior of other bimetallic systems”, highlights Jonder Morais. “Mostly, these results can be used in the “design “of new nanomaterials more efficient for various applications, such as in the petrochemical industry, in fuel cells or in the control of greenhouse gas emissions,” he concludes.
