|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Ricardo D. Rodrigues (1951-2020). Exceptional human being and true polymath.

Antonio Ricardo Droher Rodrigues (Ricardo to his colleagues and friends) passed away two years ago, on January 3, 2020. Ricardo led the projects and construction of the two synchrotron light sources built at the Brazilian National Synchrotron Light Laboratory (LNLS), an entity now belonging to the National Center for Research in Energy and Materials (CNPEM), Campinas – São Paulo state, Brazil.
Ricardo graduated as a Civil Engineer in 1974 at the Federal University of Paraná, Curitiba-UFPR, began his scientific activities in 1974 in the X-Ray Optics Group of the Physics Department at UFPR and performed his doctorate in Physics from 1976 to 1979 at King’s College, University of London, UK. In his thesis work “X-ray optics for synchrotron radiation” Ricardo proposed and characterized nearly parallel double-crystal X-ray monochromators for suppression of harmonic components, which are now used in many synchrotron light laboratories around the world. In 1977 he carried out the tests of these monochromators in Hasylab (Hamburg), thus being the first Brazilian to use a synchrotron light source.
Soon after his return to Brazil in 1981, Ricardo actively participated in the Synchrotron Radiation Project (PRS/CNPq) developed at the Brazilian Center for Research in Physics (CBPF), Rio de Janeiro, from 1980 to 1985. The PRS was the precursor project that led to the creation of LNLS in 1986. As part of the PRS activities, a group of Brazilian scientists led by Ricardo did a three-month stay at Stanford Synchrotron Radiation Lightsource (SSRL), in Stanford, USA, during which they developed a conceptual project of a 2-3 GeV synchrotron light source, which later was the initial LNLS project.
Shortly after the LNLS was created in 1986 in Campinas, Ricardo was appointed Project Manager. During a total period of 10 years, he implemented the initial basic infrastructure, trained the young technicians and engineers of his team and developed the various actions that led to the construction and successful operation of the first light source of LNLS – with an electron energy of 1.37 GeV – called UVX. The construction of the UVX source was completed in 1996 and opened to external users from Brazil and abroad in July 1997, thus providing scientists from numerous areas of science with a modern instrumentation that only exists today in few countries in the world. At that time, UVX was the only synchrotron light source in the Southern Hemisphere and even today the LNLS is the only National Laboratory in Latin America equipped with asynchrotron light source. Ricardo’s qualities of clear leadership, extreme dedication and unquestionable competence, both scientific and technical, were of fundamental importance for the successful development of the first Brazilian synchrotron light source. In addition to Ricardo’s decisive role in the construction of the first LNLS light source, he also actively participated in the development of new scientific instrumentation for several beamlines. The UVX source operated satisfactorily and was extensively used for 22 years, until 2019, by more than 6,000 researchers, mainly Brazilians and also from other countries.
In 2001, with the UVX synchrotron source already working routinely, Ricardo decided to move away from LNLS and created the company Skedio Technologies in Campinas, where he started the production of precision industrial instrumentation and also devices of artistic interest. He remained at this company until 2009, when he received and accepted an invitation from the LNLS board to return to this institution and face the second major challenge of his professional career: the design and construction of the second Brazilian synchrotron light source (Sirius).
In 2009 Ricardo assumed the role of Sirius Project Leader with the mission of designing and building a fourth-generation 3 GeV synchrotron light source with light emission qualities much superior to those of the UVX source. At that time, the only synchrotron source in the world with this exceptional quality was in the design phase in Sweden. The construction of this modern source presented numerous engineering challenges, many of them without precedent in Brazil and abroad. However, Ricardo and his team overcame these problems by applying in many cases local solutions. Thus, the first X-ray beam produced by the 3 GeV Sirius source was emitted in December 2019. Sirius is the third state-of-the-art (fourth-generation) synchrotron light source now operating in the world, after the existing ones in Lund (Sweden) and Grenoble (France). In this way Ricardo as Sirius Project Leader achieved the ambitious goals of the project and thus won the second great challenge of his career.
Ricardo demonstrated a clear leadership capacity, extreme seriousness and recognized competence both as a physicist in the area of X-ray optics and in different areas of engineering: civil, mechanical and electrical-electronic, with emphasis on the subareas of electrical circuits, magnetics and electronics.This multi-faceted competence allowed him to efficiently act on all relevant technical aspects associated with the construction of both LNLS light sources and demonstrated his leadership respected by all his team. Notably, Ricardo was not only a respected leader, an excellent physicist and a competent engineer in several specialties, he also demonstrated artistic sensitivity and competence as a sculptor and painter. This shows that Ricardo possessed all the typical characteristics of a true polymath. That is to say, his multifaceted competences were not merely those exhibited by “generalists”, but the ones demonstrated by rare and distinguished human beings who have deep knowledge in the different areas in which they work.
Ricardo’s work was unanimously recognized by the LNLS team and user researchers who knew him. He also received formal honors from the Brazilian Society of Crystallography in 2000 and from LNLS/CNPEM on the occasion of the celebration of the 30th anniversary of the LNLS, in 2017. In 2010, he received a distinction from the Presidency of the Republic of Brazil that designated him Commander of the National Order of Scientific Merit.
I had the privilege of following Ricardo’s work for over more than 40 years. Our first meeting was at the XI Congress of the International Union of Crystallography (IUCr) held in Warsaw in August 1978, during which we talked about the new scientific possibilities opened up by the availability of synchrotron light sources, which were still in their infancy in the world at that time. From 1981 to 1986 my interaction with Ricardo was mainly at CBPF, in Rio de Janeiro, during the development of the Synchrotron Radiation Project, from 1987 to 2000 at LNLS, in Campinas, during the construction of the UVX source, from 2000 to 2009 at the company Skedio Technologies, and finally, from 2009 to 2019, again at LNLS. Our last conversation was during the last week of December 2019, in which he told me – serenely and with contained satisfaction – that the electron beam in Sirius reached its nominal 3 GeV energy and the first experiments by users were carried out. Sadly, Ricardo passed away on January 3, 2020, just a few days after having won the second big challenge of his professional life.
Two years have passed since Ricardo’s death. We physically lost an exceptional human being, a distinguished master, a brilliant physicist and engineer, and a sensitive fine artist. Ricardo’s legacies for Brazilian science are the modern Sirius synchrotron light source open to users from all areas of science from Brazil and abroad, the competent team of engineers and technicians from the LNLS that he formed and the large community of LNLS
users who benefited from the results of his work. His example of life and unique personality continue and will continue to live in the memory of all those who have had the privilege of knowing him and had followed his fruitful work. Ricardo’s death mourned Brazilian science and engineering.
After his death, LNLS/CNPEM honored Ricardo by naming its annual school on applications of synchrotron light as Ricardo Rodrigues School of Synchrotron Light, and organized, on November 9, 2020, a Ceremony of Tribute to Ricardo Rodrigues. In this ceremony, family, friends and colleagues presented emotional testimonies with memories and personal visions about Ricardo’s life. In the final part, B-MRS honored Ricardo by delivering a plaque engraved with the words: The Brazilian Society for Materials Research (SBPMat) honors Ricardo Rodrigues’ fundamental contribution to the successful development and implementation of Brazilian synchrotron light sources UVX and Sirius, which put Brazil at the forefront of materials research. All the testimonies presented (in Portuguese) at the Tribute Ceremony were recorded and can be accessed through the link https://www.youtube.com/watch?v=hrmTDdnyv9s
Aldo F. Craievich
Senior Professor
Institute of Physics
University of São Paulo
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
In the year 2021, the pandemic continued to dominate our lives, but the vaccines that science provided us in record time are playing their part. Little by little, we are leaving our virtual life, resuming face-to-face activities to incorporate this new reality.
Unfortunately, our event this year still had to be virtual. Despite that, it was possible to feel the presence of each one of you on the screen!! With each work presented, with each question asked by a student, we felt reassured that science is still well represented in our country! This gives us hope for the future – much-needed hope in the face of the enormous challenges that lie ahead.
Carl Sagan said that we have to know the past to understand the present. And past and present show us that education and science are the main basis for a future with decent living conditions and social well-being. We hope that in 2022 we will continue to fight together for these values, resisting the denialism that still tries to remain present in our society. And that we can finally share our experiences – and our science – in Foz de Iguaçu!
We wish you an excellent end of the year to all, observing all the necessary sanitary care.😊
B-MRS Executive Board
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
Scientific research carried out at the Brazilian Federal University of São Carlos (UFSCar) sheds new light on supercooled liquids and glasses – two states of matter, in the broadest sense of the expression, which still present many fundamental questions to science. In particular, the work provides strong evidence to solve an old paradox involving supercooled liquids, and opens perspectives for the production of new glassy and crystalline materials.
Supercooled liquids are those that, even at temperatures below the melting point, remain in a liquid state. The best known example is water, which freezes at 0 °C but can be kept as a supercooled liquid even after a few hours in the freezer, as shown in this video.
But not just water, any liquid can be in a supercooled state as long as the conditions that prevent the formation of the first crystal, a phenomenon called nucleation, are met. However, if nucleation does occur, the delicate balance of the supercooled liquid will break down and it will crystallize into a much more stable state. To trigger this process, just waiting for a while is sufficient, stirring the supercooled liquid or introducing a catalyst into it.
In addition to arousing the curiosity of scientists and lay people, supercooled liquids have some applications in situations where it is necessary to lower the temperature to very low levels without causing freezing (crystallization), such as, for example, the preservation of organs donated for transplantation.
In this work, the authors sought to understand the interaction between two phenomena that concur during the crystallization process of supercooled liquids: relaxation (a phenomenon that occurs spontaneously in the amorphous structure of super-cooled liquids on their way to a phase of greater stability) and crystal nucleation. For this, they used atomistic computer simulation tools, that allows describing the position of each atom of a compound as a function of time, to simulate these processes in germanium, whose melting temperature is 938 °C. Above that temperature, germanium crystals “melts”. Below it, if the conditions that prevent the nucleation of crystals are maintained, the liquid germanium does not solidify and remains as a supercooled liquid.
It all started with a paradox
The idea of studying the interaction between nucleation and structural relaxation came from Professor Edgar Dutra Zanotto in 1987, when he was a young professor at UFSCar and coordinated the Vitreous Materials Laboratory, which he had created 10 years before.
It was then that Professor Zanotto began to study the Kauzmann paradox. Published in 1948, this theoretical prediction is named after Walter Kauzmann, who was a professor at Princeton University (USA) and made important contributions to the study of supercooled glasses and liquids. The paradox states that, at a given temperature (called the Kauzmann temperature), the entropy of a supercooled liquid must equal the entropy of the crystalline phase of the same compound. In this context, if cooling continued, the supercooled liquid would end up having zero entropy at a temperature above absolute zero. To avoid this situation, which contradicts the third law of Thermodynamics, supercooled liquids should crystallize before relaxing to the vitreous state, which is a non-crystalline state, above the Kauzmann temperature.

The dilemma aroused so much interest in Zanotto that he set out to investigate whether crystallization of supercooled liquids would occur in less time than structural relaxation. However, this was not an easy task (which is why the paradox persists) and required the mastery of specific computational tools. Thus, the work only started three decades later, when two post-doctoral students specializing in molecular dynamics simulation, Azat Tipeev and Leila Separdar, joined Professor Zanotto’s research group. The new members received co-orientation from Professor José Pedro Rino, also a specialist in the technique, who is a colleague of Zanotto at UFSCar and at the Center for Research, Technology and Education in Vitreous Materials (CeRTEV). While Azat was focusing over liquid germanium, Leila was working on the same problem with other substances. Some of the results of Leila’s work are published in this article in the journal Computational Materials Science.
“Molecular dynamics simulations allow the study of crystallization and relaxation at the atomistic level, in a region of states not yet attainable by laboratory experiments, to obtain essential information about the properties of tiny crystal nuclei in an extremely short time scale and, consequently, testing nucleation and relaxation theories,” explains post-doc Azat, of Russian nationality, who met Professor Zanotto in 2012 at an event on crystallization of glass and liquids in Germany and came for the first time to Brazil in 2015 to participate in the Advanced School of Glass and Vitroceramics organized by Zanotto with funding from FAPESP.
Based on the simulations, the authors determined the structural relaxation and stresses times and compared them with the formation times of the first crystal nucleus at different temperatures. “We found that these curves intersected at the so-called kinetic spinodal temperature, establishing a temperature region where the (strong) interference of relaxation in nucleation must be considered by theoretical models to adequately describe the dynamics of experimental nucleation,” summarize the authors.
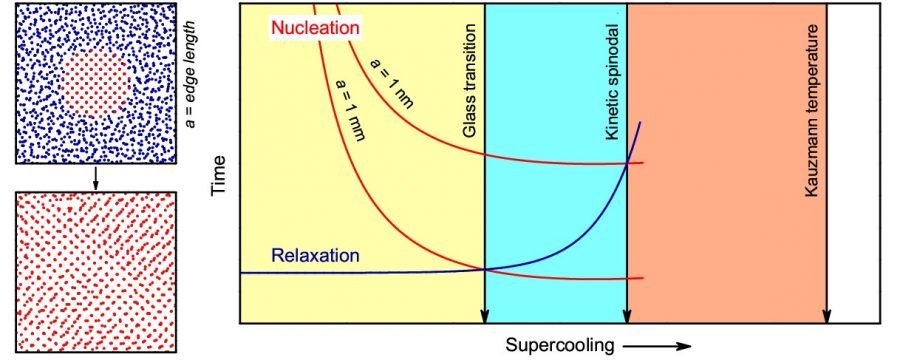
Furthermore, the work provided solid evidence for the resolution of the Kauzmann paradox. “Our work demonstrated that the supercooled germanium liquid crystallizes before reaching the Kauzmann temperature, avoiding the entropy catastrophe,” says Azat, who is the first author of the article reporting this research in the journal Acta Materialia.
The new articles co-authored by Azat, Leila and Pedro Rino are part of the vast scientific production that Professor Zanotto and his collaborators have in the area of glass materials. “The crossing of relaxation and nucleation times above the Kauzmann temperature is significantly important to clarify the processes and dynamics of vitrification and crystallization and the very nature of the glassy state,” says Zanotto.
The work was carried out with funding from FAPESP.
Scientific paper reference: Unveiling relaxation and crystal nucleation interplay in supercooled germanium liquid. Azat O. Tipeev, José P. Rino, Edgar D. Zanotto. Acta Materialia. Volume 220, November 2021, 117303. https://doi.org/10.1016/j.actamat.2021.117303.
Author contact: Edgar Dutra Zanotto – dedz@ufscar.br
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
 Professor Daniel Mario Ugarte (UNICAMP), founding member of B-MRS, was sworn in as a fellow of TWAS (The World Academy of Sciences) in a virtual ceremony held on November 4 within the 15th TWAS general conference.
Professor Daniel Mario Ugarte (UNICAMP), founding member of B-MRS, was sworn in as a fellow of TWAS (The World Academy of Sciences) in a virtual ceremony held on November 4 within the 15th TWAS general conference.
The election of Ugarte as a member, in the field of Physics, took place in 2019, but the inauguration ceremony was postponed due to the Covid-19 pandemic.
TWAS fellows are scientists whose contributions to science meet international standards of excellence and who work in or promote research in developing countries.
More information on TWAS member election: https://twas.org/directory/regulations.