
With molecules similar to those that nature uses to make up proteins, Professor Junbai Li produces nanomaterials for biomedical applications. More precisely, the Chinese scientist uses an amino acid known as diphenylalanine as the basic unit to form structures based on peptides (amino acid chains) by means of self-assembling processes. Although these processes occur spontaneously, Prof. Li has his own recipes in order to control the format of the resulting structures.
The fabrication and applications of these self-assembled nanomaterials will be the subject of Professor Li’s plenary lecture at the XVII B-MRS Meeting, entitled “Molecular Assembly of Peptide Based Materials towards Biomedical Application”.
Junbai Li is a professor at the Institute of Chemistry at the Chinese Academy of Sciences. He is the author of over 280 articles published in international journals and 8 book chapters, and owner of 20 authorized patents. He is also the editor of 5 books. His scientific production has 10.100 citations and his index h is 55. Li serves as editor-in-chief of the journal Colloids & Surfaces A (Elsevier) and editor of the self-assembling section in Current Opinion in Colloid & Interface Science (Elsevier). Junbai Li received his Ph.D. in Chemistry from the University of Jilin (China) in 1992 and held postdoctoral studies at the Max Planck Institute for Colloids and Interfaces (Germany) from 1994 to 1996.
See our mini interview with Professor Li.
B-MRS Newsletter: – What do you think are the most promising applications of peptide-based self-assembled materials and why?
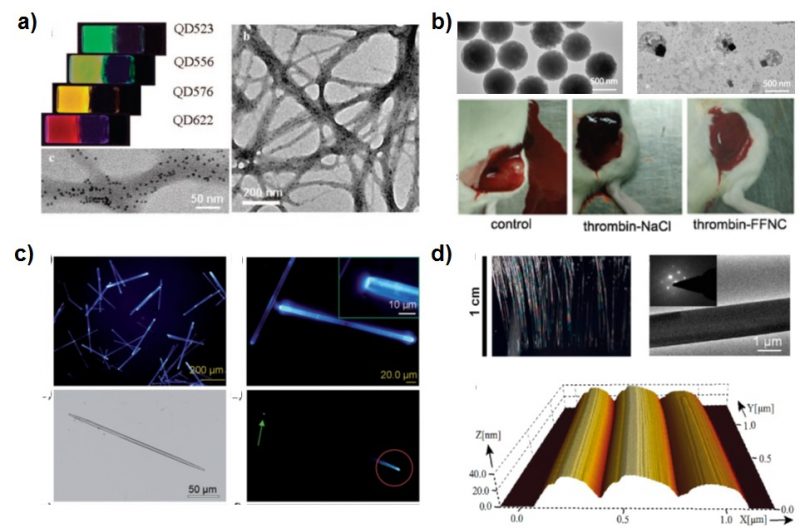
Junbai Li: – Peptide-based nanostructures have attracted considerable attention owing to their biocompatibility, capability of molecular recognition, and well-defined structures. Firstly, the cationic dipeptides self-assemble into nanotubes at physiological pH values, and these cationic dipeptide nanotubes can also rearrange to form vesicles upon dilution. Furthermore, they can traverse cell membranes and be absorbed by the cells upon spontaneous conversion into vesicles. With such a surface highly positive charged property, peptide-based self-assembled materials can effectively be used for the genic transfer and delivery. Secondly, the traced quantum dots (QDs) can be well distributed in a peptide-based gel against QDs aggregation and oxidation to improve the stability for bioimaging and biosensor.
B-MRS Newsletter: – We want to know more about your work. Please choose two papers/ patents of your own (your favorites) and briefly describe them, as well as share the references.
Junbai Li: – Our group have worked on the self-assembly of aromatic dipeptides for a long time. We find that cryogenic treatment at 77 K enabled the tunable transition of a self-assembled diphenylalanine organogel into a hexagonal crystal and form a well-defined chiral crystal structure. These assemblies exhibit enhanced emission. (X.C. Liu, et al. Angew. Chem. Int. Ed. 2017, 56, 2660-2663).https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.201612024
Another work is under light illumination, a long-lived photoacid generator releases a proton and mediates the dissociation of dipeptide-based organogel, resulting in sol formation. Under darkness, the photoswitchable moiety entraps a proton to lead to the gel regeneration. It opens a new possibility for the light-controlled phase transition of peptide-based biomaterials. (X. B. Li, et. al. Angew. Chem. Int. Ed. 2018, 57, 1903 -1907). https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.201711547
For more information on this speaker and the plenary talk he will deliver at the XVII B-MRS Meeting, click on the speaker’s photo and the title of the speech here https://www.sbpmat.org.br/17encontro/home/
