
Perovskites are very promising semiconductor materials for use in solar cells and other optoelectronic devices, but they have a limitation that impairs the useful life of their applications, their low stability. In this context, humidity can be considered the enemy of perovskites, as it is one of the main factors that accelerate the degradation of these materials.
Now, a discovery by researchers from the Brazilian Federal University of ABC (UFABC) brings a very different perspective on the interaction of water with perovskites. With a process based on the simple hydration of three-dimensional perovskite structures, the scientists were able to generate two-dimensional (sheet) and one-dimensional (wire) structures, which, in addition to being more stable than 3D structures, have different properties.
“In controlled amounts, the presence of water can be extremely beneficial for the synthesis of new structures in perovskites”, says André L. M. Freitas, postdoctoral fellow and author, together with Prof. José A. Souza, of the paper that reports this work in the Journal of Materials Chemistry C.
The authors produced micrometric cubes of MAPbBr3, a hybrid material (organic – inorganic) from the perovskite family, which is usually used in the academic environment in solar cells and LEDs. In this material, the inorganic part forms an octahedron, where the central atom (Pb) is surrounded by six halides (Br). “The octahedrons are extremely important in these materials, as they are directly responsible for the physical properties of the material”, explains the postdoc.
To generate the wires, the microcubes were placed in water. To produce the sheets, an organic molecule was added to water. In contact with the liquid, almost instantly, the material lost its crystalline structure, forming what the UFABC duo called an intermediate phase. “We faced a great challenge when analyzing this phase, as the process occurs quickly and many times we had to repeat the experiments to properly characterize it”, says Freitas.
Subsequently, when subjecting the material to drying, the water molecules were expelled from the structure and an interesting conversion took place: the material once again had a perovskite structure, but with a morphology and dimension different from the original ones. “That was one of our main contributions; we were able to control the recrystallization process and obtain different structures and dimensions”, says Freitas.
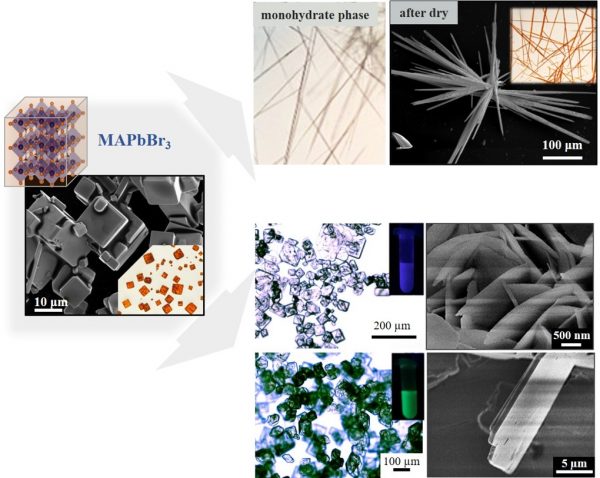
In the formation of the wires, the process inhibited the growth of the crystalline structure in two directions. In the case of the sheets, the organic molecule added to the water acted as a barrier to the growth of the material in one direction, as if it was a spacer between the layers of octahedra, giving rise to a new structure of the 2D perovskite family. Especially in the 2D structures, this confinement, which occurred on a very small scale, restricted the movement of electrons, resulting in very pronounced quantum effects which modified the electrical and optical properties of the material.
“Our observation revealed that dissolved or degraded material can recrystallize into interesting structures”, summarizes Freitas. According to the authors, by exploring the hydration process of perovskites, the work brings new perspectives to advance the understanding of the degradation and recrystallization of these materials, as well as to understand optical properties such as those involving the dynamics of the exciton (the electron – hole pair generated from the excitation of light in the semiconductor).
The work presents a simple, scalable and sustainable process to produce low-dimensional perovskites with controlled properties. The results could impact both application development and the study of the fundamentals of these versatile materials.
The research received financial support from Brazilian agencies FAPESP and CNPq.
Scientific paper reference: Water-induced dimensionality conversion from 3D perovskites to microwires and 2D hybrid halide perovskites. Andre L. M. Freitas and Jose A. Souza. J. Mater. chem. C, 2023, 11, 6651. https://doi.org/10.1039/D3TC00593C.
Authors contact: joseantonio.souza@ufabc.edu.br, andre_luizmf@yahoo.com.br.
