A team of scientists from Brazilian institutions has increased by about 30 times the capacity of a semiconductor material to produce hydrogen by means of water photolysis, a process that consists of dividing the water molecule using light as the only source of energy. The advance contributes to the development of efficient ways to generate green hydrogen, which is the fuel produced using renewable and clean energy.
For photolysis to take place, it is necessary to have photocatalysts suspended in water. A photocatalyst is a semiconductor capable of absorbing light and, from there, generating the charges (electrons and holes) that are necessary to dissociate water molecules (H2O) into hydrogen (H2) and oxygen (O2) though oxidation and reduction reactions. Furthermore, the material must be stable in an aqueous environment.
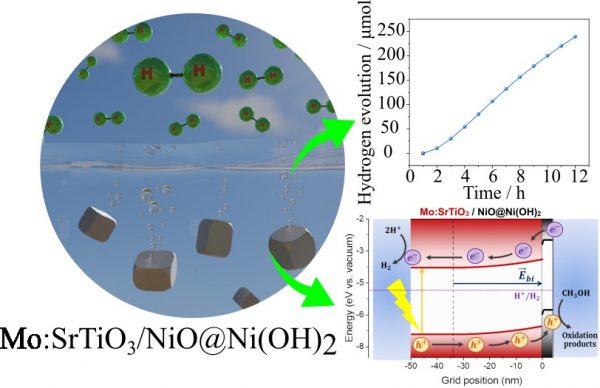
“Strontium titanate (SrTiO3) is one of the main semiconductor materials applied to photolysis for the production of green hydrogen, as it meets the physicochemical requirements for oxidizing and reducing the water molecule,” says Professor Renato Vitalino Gonçalves (IFSC-USP) , corresponding author of the article that reports this research in ACS Applied Energy Materials. “However, this material has some intrinsic characteristics that limit its photocatalytic potential, such as, for example, its wide bandgap of ~3.2 eV, which restricts its optical absorption to the UV region, which corresponds to only 4% of the solar spectrum”, completes the scientist. Another limitation of this material, common to all semiconductors, is the rapid recombination of electrons and holes, which prevents these charges from flowing freely and promoting oxidation and reduction reactions.
Thus, the Brazilian team, led by Professor Gonçalves, decided to modify strontium titanate to increase its efficiency in photolysis. Initially, the researchers doped the semiconductor with the transition metal molybdenum (Mo) and obtained disaggregated cubic particles with well-defined faces. The unconventional dopant was responsible for making the material capable of absorbing light in the visible region, which represents around 43% of the solar spectrum.
In a second moment, the authors of the work deposited nickel nanoparticles of around 2 nm on the surface of the particles. The result was a junction of two types of semiconductors: Mo:SrTiO3, n-type, and NiO@Ni(OH)2, p-type. “In this new configuration, the photogenerated holes are directed to the NiO@Ni(OH)2 structure, while the electrons migrate to the Mo:SrTiO3 surface, resulting in better charge separation and, consequently, a reduction in the recombination rate of electrons and holes”, explains Gonçalves.
The photocatalysts were placed in suspension in an aqueous solution with 20% methanol as a sacrificial agent – a widely used strategy to increase hydrogen production and also generate high-value by-products for the chemical industry. “When mixed with water, which oxidation is slow, this alcohol is preferentially oxidized”, says Professor Gonçalves. “Even though, the H2 is produced from the reduction of the water molecule and not as a by-product of methanol oxidation”, he adds.
By increasing the absorption of light and decreasing the loss of photogenerated charges, the enhanced material presented an excellent result in the production of hydrogen by photolysis: an increase of its photocatalytic activity of about 30 times compared with the pure semiconductor.
Brazilian scientific cooperation
This scientific work was led by Professor Renato Vitalino Gonçalves, who coordinates the Nanomaterials and Advanced Ceramics Group (NaCA) and the Artificial Photosynthesis and Nanomaterials Laboratory (LAPNano) at IFSC-USP. The synthesis of materials and the study of their structural, optical and electronic properties, as well as their photocatalytic performance for the production of green hydrogen were developed at IFSC-USP, within the doctoral research of Higor Andrade Centurion, supervised by Professor Gonçalves.
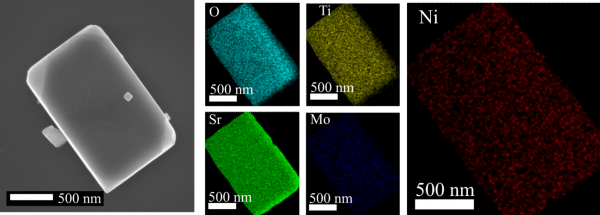
The identification and characterization of the nickel nanoparticles in the material was carried out in collaboration with a team from UFABC and LNNano-CNPEM, formed by Professor Flávio Leandro de Souza, postdoctoral student Ingrid Rodriguez-Gutierrez and researcher Jefferson Bettini. In collaboration with Professor Liane M. Rossi (IQ-USP), nickel was quantified using the flame atomic absorption spectroscopy technique.
In addition, with the collaboration of Professor Heberton Wender (UFMS) it was possible to carry out photoluminescence measurements that corroborated the suppression of recombination of charges photogenerated by the formation of the p – n junction.
Finally, computer simulations that made it possible to understand the behavior of the materials were carried out with Professor Matheus M. Ferrer, from UFPel, and Master’s student Lucas Gabriel Rabelo, from IFSC-USP, who also received guidance from Professor Gonçalves.
The work was funded mainly by the São Paulo research foundation (FAPESP) and, through the RCGI, by FAPESP/Shell. It also had financial support from the research foundation of Rio Grande do Sul (FAPERGS).

Paper reference: Constructing Particulate p−n Heterojunction Mo:SrTiO3/NiO@Ni(OH)2 for Enhanced H2 Evolution under Simulated Solar Light. Higor A. Centurion, Lucas G. Rabelo, Ingrid Rodriguez-Gutierrez, Mateus M. Ferrer, Jefferson Bettini, Heberton Wender, Liane M. Rossi, Flavio L. Souza, and Renato V. Gonçalves. ACS Appl. Energy Mater. 2022, 5, 12727−12738. https://doi.org/10.1021/acsaem.2c02337.
Corresponding author contact: rgoncalves@ifsc.usp.br.

João Arnaldo Lessa Jordão
É possível uma pequena indústria química conseguir produzir em pequena escala o hidrogênio verde ?
A partir desse processo totalmente inovador e econômico com uma pequena estrutura com energia solar já implantada para tornar a extração viável.