Experts say that, to date, there is nothing better than a natural bone to help regenerate another natural bone. In fact, despite the enormous advances in the area of biomaterials, there is still no synthetic material that works as well as autogenous bone graft (bone extracted from the patient himself) to encourage bone tissue regeneration – a process that occurs in our body regularly and spontaneously, but it is necessary to stimulate this through graft implantation when we experience noticeable bone loss due to trauma or disease. In this process, the basic unit of bone formation is the mineralized collagen fibril, a collagen cylinder formed by a group of cells called osteoblasts, which is filled and coated with calcium phosphates during the “biomineralization” process.
A study carried out by researchers from Brazil with collaborators from France took an important step in this context. Using a strategy inspired by nature, the scientific team produced, in the laboratory, a material that is very similar to mineralized collagen fibrils, in shape, size and structure. Collagen structures, which are expensive and difficult to handle proteins, were not used in this research. Instead, the researchers produced tubes (about 200 nm in diameter) which are very similar to fibrils, but composed of calcium phosphate crystals, the main inorganic component of bones, and strontium, an element used to treat osteoporosis, which helps reduce bone tissue loss and increase bone formation.
In in vitro tests, the material showed that it is not toxic to cells and that it generates the necessary conditions for bone tissue formation. In addition, it showed the ability to release strontium ions for long periods in controlled doses – an essential parameter for the long-term development of safe therapies, since in excess it can lead to the weakening of bones.
Strategy
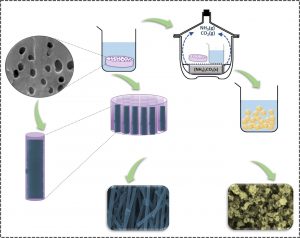
To produce the material having a cylindrical structure of controlled dimensions, the team employed a strategy that is used by several living organisms to generate teeth, shells and bones: physical confinement, which is nothing more than the use of a mold to induce a material to follow a specific morphology and size.
The mold used was a polycarbonate membrane, commercially available, which has cylindrical pores of 200 nm in diameter, similar in size to bone collagen fibrils. The membrane was submerged for twelve hours in a solution containing phosphate, calcium and strontium, which penetrated the pores. After drying the membrane in the presence of compounds that triggered mineralization, the polycarbonate was dissolved, allowing to separate and analyze the material that had formed inside the pores.
“By delimiting the physical environment in which the nucleation and growth processes of the mineral occur, we obtained nanotubes with highly controlled morphology, composition and size,” says Ana Paula Ramos, professor at the University of São Paulo (USP), campus Ribeirão Preto, and corresponding author of a recently published article reporting the study.
The same procedure, but without the use of molds, resulted in agglomerated spherical nanoparticles with very different characteristics from the collagen fibrils that were sought to emulate.
Surprise
The research was carried out within the PhD in Chemistry of Camila Bussola Tovani, which was carried out with funding from FAPESP, supervised by Professor Ana Paula Ramos, and defended this year at USP, campus Ribeirão Preto.
The initial idea of the project was to understand how strontium ions, used in the treatment of osteoporosis, acted on the bone mineralization mechanism. As this process, in the body, occurs in a situation of confinement, limited by the framework formed by the collagen fibrils, Camila and her advisor decided to use a mold to study how the calcium phosphate mineralization occurs in the presence of strontium ions.
“During the structural characterization, we found high similarity between the particles obtained and the mineralized collagen fibrils that form the bones, which encouraged us to conduct biological investigations”, says the professor. “What really surprised us, was the possibility of controlling particle properties such as mineral phase, morphology and size, by simply changing the medium in which precipitation occurred,” adds Ana Paula, who suggests that the same strategy can be applied to synthesize other inorganic particles for different applications in which the control of physicochemical properties is essential.
The nanotubes were produced and characterized in the Physical and Chemical Laboratory of Surfaces and Colloids, coordinated by Professor Ana Paula. Biological tests were carried out in other laboratories at USP campus in Ribeirão Preto, through collaborations with Professor Pietro Ciancaglini and Professor Sandra Fukada.
The study also benefited from two collaborations by Professor Ana Paula with researchers from France. The first, with the researcher Alexandre Gloter (Université Paris-Saclay), made it possible to investigate the formation mechanism and to characterize the nanotubes by advanced spectroscopy and microscopy techniques. The second, with researcher Nadine Nassif (Sorbonne Université), helped to understand the bone mineralization processes. “It is interesting that the initial contact with Dr. Alexandre Gloter took place in Brazil during TEM Summer School, organized at CNPEM by LNNano, and that the BEPE-FAPESP scholarship allowed Camila Tovani to stay at the Chimie de la Matiere Condensée laboratory at Sorbonne Université, under the supervision of Dr. Nadine Nassif for one year,” says Ana Paula Ramos. “The investment of Brazilian funding agencies in internationalization, both in bringing and sending researchers abroad, had an important impact on this research,” she concludes.
From the laboratory to the market
According to Professor Ana Paula, after conducting an investigation to validate the performance of nanotubes in animal models, the material will be able to be used to locally fill small bone defects. The nanotubes could also be incorporated into polymeric matrices that are used in orthopedic and cranio-maxillofacial surgery to replace, fill or repair bone defects caused by infections, injuries and neoplasms. In addition, the material could be incorporated into toothpaste formulations for the treatment of tooth hypersensitivity, since some toothpastes used for this purpose have strontium in their composition.
“The biggest challenge to make the particles as product is to find companies in the industry interested in the technology, which would allow us to develop formulations in the short term,” says the researcher.

[Paper: Strontium Calcium Phosphate Nanotubes as Bioinspired Building Blocks for Bone Regeneration. Camila B. Tovani, Tamires M. Oliveira, Mariana P. R. Soares, Nadine Nassif, Sandra Y. Fukada, Pietro Ciancaglini, Alexandre Gloter, and Ana P. Ramos. ACS Appl. Mater. Interfaces 2020, 12, 39, 43422–43434. doi.org/10.1021/acsami.0c12434.]
