[Paper: NiMo–NiCu Inexpensive Composite with High Activity for Hydrogen Evolution Reaction. Hugo L. S. Santos, Patricia G. Corradini, Marina Medina, Jeferson A. Dias, and Lucia H. Mascaro. ACS Appl. Mater. Interfaces. 2020, 12, 15, 17492–17501. https://doi.org/10.1021/acsami.0c00262]

Hydrogen, whose combustion does not generate polluting emissions, is currently recognized as the most promising alternative to fossil fuels. However, there are still challenges in the sustainable production of this fuel, such as the development of a production process that is at the same time clean, economical and efficient.
Water electrolysis is one of the processes that can fit in these conditions. As the name implies, the process consists of the division of the water molecule, based on the action of an electric current. As a result, this simple reaction produces hydrogen gas and oxygen gas. However, to become a viable industrial process, water electrolysis needs catalysts that accelerate hydrogen production without significantly increasing its costs.
A work by a scientific team from the Brazilian Federal University of São Carlos (UFSCar), recently reported in an article in ACS Applied Materials & Interfaces, brings a contribution to overcome this challenge. “The main contribution of our work is to obtain a catalyst with significant performance improvement for the production of hydrogen from water electrolysis,” summarizes Lucia Helena Mascaro, professor at UFSCar and corresponding author of the paper.
The new catalyst is a film that can be deposited on the surface of the electrode used in the electrolysis process, which is why it is called an electrocatalyst. The electron transfer occurs in this negatively charged electrode, which causes hydrogen to detach from the water molecule.
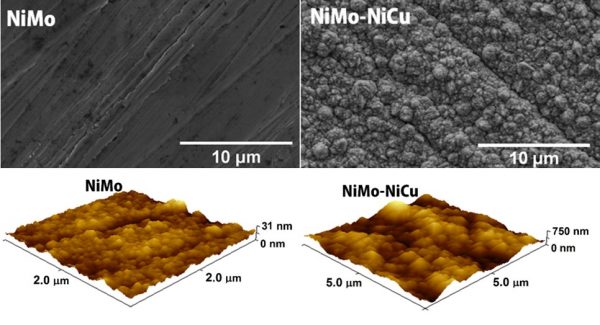
While some electrocatalysts are made with noble metals, such as platinum, the Brazilian team looked for a material based on more abundant and economical elements, but which had good electrocatalytic activity and durability. The final option was for a metallic alloy composed of nickel (Ni), molybdenum (Mo) and copper (Cu), which presented two defined regions (phases), NiMo and NiCu, both with crystalline structures, composing a composite material.
“The NiMo-NiCu material showed excellent electrocatalytic activity, robustness, low toxicity, wide availability and economic viability for the hydrogen release reaction, called hydrogen evolution reaction. This material was easily obtained by electroplating, which is a simple, inexpensive and scalable technique, on a carbon steel substrate, without the need for any other treatment,” says Mascaro.
The main secret of the good performance of the film as an electrocatalyst is dependent on surface roughness, caused by the presence of copper. In relative terms, the NiMo-NiCua film presented roughness more than thirty times greater than the NiMo film, which is widely used to catalyze the release of hydrogen in water electrolysis.
In fact, the topography of a rough surface provides many more opportunities for the water molecule to come in contact with the catalyst and then the electron transfer that generates hydrogen release occurs. “The increase in the film’s roughness implies the formation of a greater amount of hydrogen for the same geometric area of the catalyst,” explains Professor Mascaro. “In addition, the NiMo-NiCu composite showed high stability during prolonged electrolysis,” she adds.
In search of the most suitable material
The genesis of the research dates back to 2012, when Professor Lucia Helena Mascaro and her team from at the Interdisciplinary Laboratory of Electrochemistry and Ceramics (LIEC) set out to find a material that would improve the efficiency of hydrogen production by water hydrolysis, and that could be produced through electrodeposition – a fast and viable process at an industrial scale, in which Professor Mascaro and her group have extensive expertise.
After developing some coatings with nickel and iron alloys, and making a thorough review of the scientific literature, the group came to the conclusion that a nickel, molybdenum and copper (Ni-Mo-Cu) alloy would be promising in terms of electrocatalytic activity and robustness, says the scientist. The UFSCar team then decided to add copper to the Ni-Mo alloy.
After defining the material, the team immersed in studying the conditions of the electroplating process. In fact, a key point for electrodeposition to generate the expected results is to correctly define the composition of the solution in which the process occurs. In this solution, also known as “electrodeposition bath,” the metals that will be deposited in the form of salts are found.
Thus, the team produced films with different concentrations of copper, characterized all of them and verified the performance of each one as an electrocatalyst in the release of hydrogen. At the end of the study, the researchers were able to safely determine which of the “recipe” had been the most successful.
The work was mainly carried out within Hugo Leandro Sousa Dos Santos’s master’s dissertation, supervised by Professor Mascaro and defended in 2018 in the Graduate Program in Chemistry at UFSCar. The research was funded by FAPESP, including through the Functional Materials Development Center (CMDF), CAPES and CNPq, and involved two UFSCar laboratories, LIEC and the Laboratory of Synthesis and Formulation Ceramic (LaFSCer), of the Materials Engineering Department.


Marcelo
Adorei a expertise da equipe pois buscava lá fora, material mas com tecnica nivel nasa ,muito contrariado. Ai vejo este brilhante anúncio, sabia que um dia brilhantes brasileiros me ajudaria mas de forma tão simples e surpriende , congratulações a todos mas ,é só o início.
Yvonne P. Mascarenhas
Parabéns!