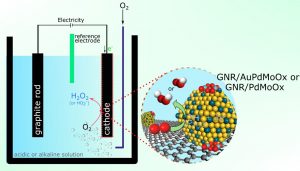
Hydrogen peroxide (H2O2) is a compound widely used, especially as a bleach or antiseptic in the production of pulp and paper, in cleaning, pharmacy and beauty products and in the treatment of wastewater, among other applications. With a large and growing market, the production of hydrogen peroxide has the challenge of becoming more sustainable, using methods that are environmentally friendly and allow the compound to be obtained in the same place where it will be used, reducing risks, costs and the environmental impact of transport. In this scenario, producing hydrogen peroxide in electrochemical generators using basically water, air and electricity is a promising path, which some companies are already treading. However, the success of this process largely depends on having efficient, stable and low-cost catalysts.
In a recently published scientific article, a team made up of researchers from the Brazilian Federal University of Mato Grosso do Sul (UFMS), Federal University of Grande Dourados (UFGD), and the São Carlos Institute of Chemistry (IQSC-USP) made a contribution in this regard. They developed catalysts based on graphene nanoribbons and metallic nanoparticles and studied in detail their performance in the electrochemical production of hydrogen peroxide. In addition to showing that these catalysts significantly improve reaction efficiency, equating to the best conventional catalysts in some aspects, the study advanced the understanding of fundamental phenomena that open possibilities to continue optimizing the electrocatalytic production of hydrogen peroxide.
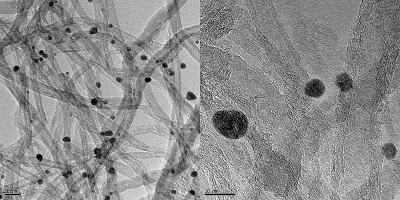
“We proposed the synthesis of catalysts with a low content of noble metal (≤6.4% by mass), with high catalytic efficiency and high durability for the electrochemical production of H2O2”, says Professor Gilberto Maia (UFMS), co-author of the article. Indeed, noble metals such as gold and palladium are known for their catalytic properties but have the disadvantage of cost. “Our catalysts were built from oxides of molybdenum, gold and palladium, which together form nanoparticles anchored on the surface of graphene nanoribbons”, describes Maia.
The team tested the efficiency of the catalysts in terms of generating hydrogen peroxide through the two-electron oxygen reduction reaction (ORR-2e– ), in which one molecule of oxygen, two hydrogen cations and two electrons form one molecule of hydrogen peroxide. Mainly, the researchers tested, with very positive results, the activity of the catalyst (its ability to increase the rate of reaction), its selectivity (its ability to direct the reaction towards a certain product, in this case, hydrogen peroxide) and its stability (the ability to maintain its properties over time).
“The results we obtained showed that the improved catalytic activity for ORR-2e– was promoted by a combination of factors including geometry, palladium content, interparticle distance and active site blocking effects, while the electrochemical stability of the catalysts may have been enhanced by the presence of molybdenum”, says Professor Maia.
The work was developed within a collaboration between researchers from the Institute of Chemistry at UFMS and the Environmental Electrochemistry Research Group at IQSC-USP, who have been working together on the synthesis, characterization and application of electrocatalytic materials. According to the authors, the main idea and the first combinations of synthesis emerged as an offshoot of the doctoral thesis by Guilherme Fortunato, which was supervised by Professor Gilberto Maia and was defended in UFMS in 2019. The work continued and finalized within the postdoctoral research of Fortunato, carried out at IQSC under the supervision of Professor Marcos Lanza.
The research was funded by Brazilian federal and state agencies Capes, CNPq, FAPESP and FUNDECT-MS.

Paper reference: Using Palladium and Gold Palladium Nanoparticles Decorated with Molybdenum Oxide for Versatile Hydrogen Peroxide Electroproduction on Graphene Nanoribbons. Guilherme V. Fortunato, Leticia S. Bezerra, Eduardo S. F. Cardoso, Matheus S. Kronka, Alexsandro J. Santos, Anderson S. Greco, Jorge L. R. Júnior, Marcos R. V. Lanza, and Gilberto Maia. ACS Applied Materials & Interfaces 2022 14 (5), 6777-6793. DOI: 10.1021/acsami.1c22362.
Corresponding authors contact: g.fortunato@usp.br and gilberto.maia@ufms.br.
