Scientific research carried out at the Brazilian Federal University of São Carlos (UFSCar) sheds new light on supercooled liquids and glasses – two states of matter, in the broadest sense of the expression, which still present many fundamental questions to science. In particular, the work provides strong evidence to solve an old paradox involving supercooled liquids, and opens perspectives for the production of new glassy and crystalline materials.
Supercooled liquids are those that, even at temperatures below the melting point, remain in a liquid state. The best known example is water, which freezes at 0 °C but can be kept as a supercooled liquid even after a few hours in the freezer, as shown in this video.
But not just water, any liquid can be in a supercooled state as long as the conditions that prevent the formation of the first crystal, a phenomenon called nucleation, are met. However, if nucleation does occur, the delicate balance of the supercooled liquid will break down and it will crystallize into a much more stable state. To trigger this process, just waiting for a while is sufficient, stirring the supercooled liquid or introducing a catalyst into it.
In addition to arousing the curiosity of scientists and lay people, supercooled liquids have some applications in situations where it is necessary to lower the temperature to very low levels without causing freezing (crystallization), such as, for example, the preservation of organs donated for transplantation.
In this work, the authors sought to understand the interaction between two phenomena that concur during the crystallization process of supercooled liquids: relaxation (a phenomenon that occurs spontaneously in the amorphous structure of super-cooled liquids on their way to a phase of greater stability) and crystal nucleation. For this, they used atomistic computer simulation tools, that allows describing the position of each atom of a compound as a function of time, to simulate these processes in germanium, whose melting temperature is 938 °C. Above that temperature, germanium crystals “melts”. Below it, if the conditions that prevent the nucleation of crystals are maintained, the liquid germanium does not solidify and remains as a supercooled liquid.
It all started with a paradox
The idea of studying the interaction between nucleation and structural relaxation came from Professor Edgar Dutra Zanotto in 1987, when he was a young professor at UFSCar and coordinated the Vitreous Materials Laboratory, which he had created 10 years before.
It was then that Professor Zanotto began to study the Kauzmann paradox. Published in 1948, this theoretical prediction is named after Walter Kauzmann, who was a professor at Princeton University (USA) and made important contributions to the study of supercooled glasses and liquids. The paradox states that, at a given temperature (called the Kauzmann temperature), the entropy of a supercooled liquid must equal the entropy of the crystalline phase of the same compound. In this context, if cooling continued, the supercooled liquid would end up having zero entropy at a temperature above absolute zero. To avoid this situation, which contradicts the third law of Thermodynamics, supercooled liquids should crystallize before relaxing to the vitreous state, which is a non-crystalline state, above the Kauzmann temperature.

The dilemma aroused so much interest in Zanotto that he set out to investigate whether crystallization of supercooled liquids would occur in less time than structural relaxation. However, this was not an easy task (which is why the paradox persists) and required the mastery of specific computational tools. Thus, the work only started three decades later, when two post-doctoral students specializing in molecular dynamics simulation, Azat Tipeev and Leila Separdar, joined Professor Zanotto’s research group. The new members received co-orientation from Professor José Pedro Rino, also a specialist in the technique, who is a colleague of Zanotto at UFSCar and at the Center for Research, Technology and Education in Vitreous Materials (CeRTEV). While Azat was focusing over liquid germanium, Leila was working on the same problem with other substances. Some of the results of Leila’s work are published in this article in the journal Computational Materials Science.
“Molecular dynamics simulations allow the study of crystallization and relaxation at the atomistic level, in a region of states not yet attainable by laboratory experiments, to obtain essential information about the properties of tiny crystal nuclei in an extremely short time scale and, consequently, testing nucleation and relaxation theories,” explains post-doc Azat, of Russian nationality, who met Professor Zanotto in 2012 at an event on crystallization of glass and liquids in Germany and came for the first time to Brazil in 2015 to participate in the Advanced School of Glass and Vitroceramics organized by Zanotto with funding from FAPESP.
Based on the simulations, the authors determined the structural relaxation and stresses times and compared them with the formation times of the first crystal nucleus at different temperatures. “We found that these curves intersected at the so-called kinetic spinodal temperature, establishing a temperature region where the (strong) interference of relaxation in nucleation must be considered by theoretical models to adequately describe the dynamics of experimental nucleation,” summarize the authors.
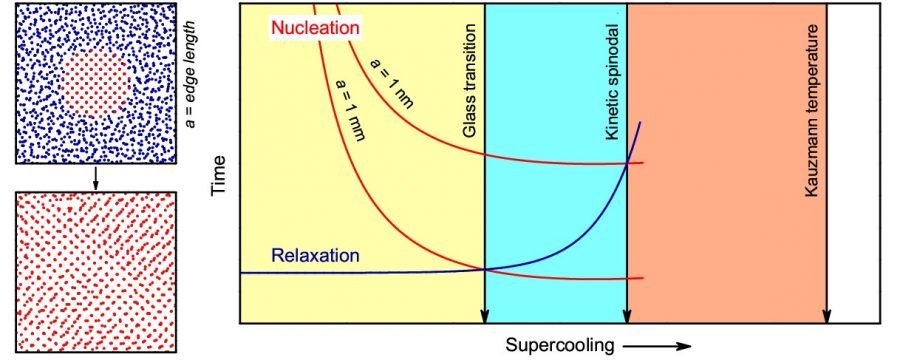
Furthermore, the work provided solid evidence for the resolution of the Kauzmann paradox. “Our work demonstrated that the supercooled germanium liquid crystallizes before reaching the Kauzmann temperature, avoiding the entropy catastrophe,” says Azat, who is the first author of the article reporting this research in the journal Acta Materialia.
The new articles co-authored by Azat, Leila and Pedro Rino are part of the vast scientific production that Professor Zanotto and his collaborators have in the area of glass materials. “The crossing of relaxation and nucleation times above the Kauzmann temperature is significantly important to clarify the processes and dynamics of vitrification and crystallization and the very nature of the glassy state,” says Zanotto.
The work was carried out with funding from FAPESP.
Scientific paper reference: Unveiling relaxation and crystal nucleation interplay in supercooled germanium liquid. Azat O. Tipeev, José P. Rino, Edgar D. Zanotto. Acta Materialia. Volume 220, November 2021, 117303. https://doi.org/10.1016/j.actamat.2021.117303.
Author contact: Edgar Dutra Zanotto – dedz@ufscar.br

Edgar Dutra Zanotto
Obrigado pela excelente, bem escrita, matéria, Verónica!